The ribosome serves as a fundamental element in biological processes, acting as the cellular framework responsible for synthesizing proteins. These proteins are crucial for the structure and function of all living organisms. Through a detailed understanding of ribosomal operations and the environment within which they function, researchers can unlock significant insights into cellular biology. A recent study from the University of Tsukuba paves the way for such advancements, providing a pioneering model that simulates the internal environment of a ribosome.
Key Insights from the Research
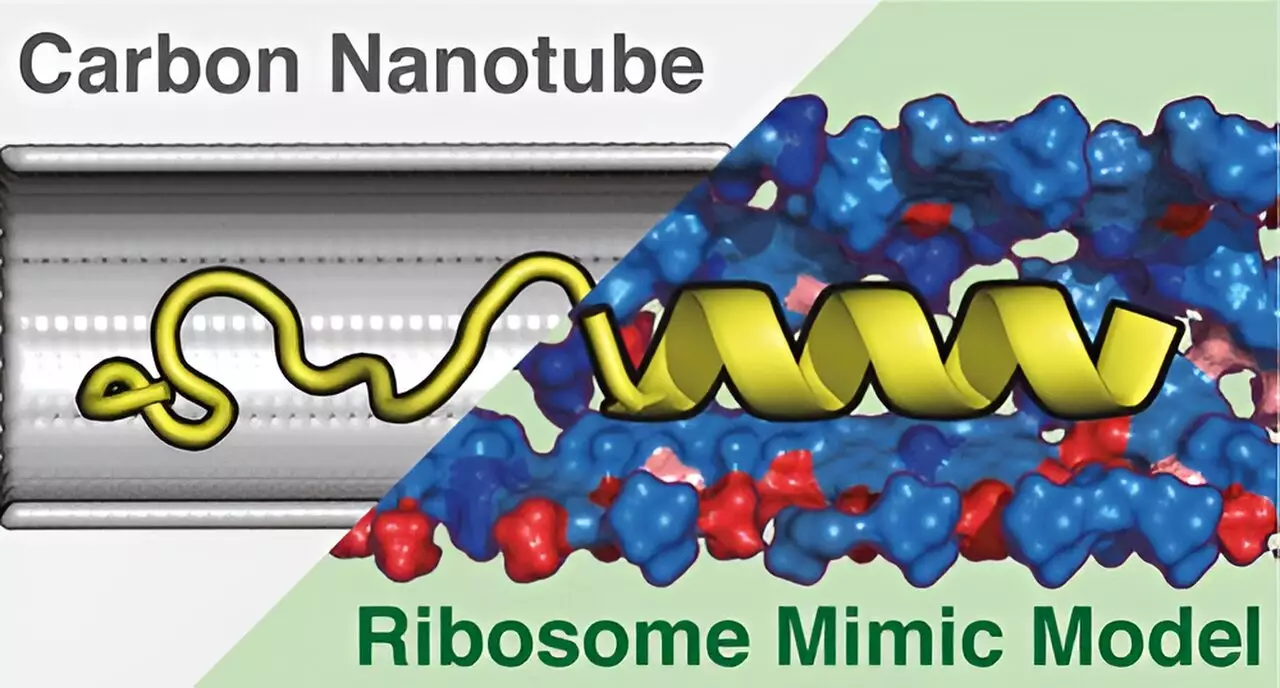
The research emphasizes the importance of the ribosome tunnel, a critical passage through which proteins are synthesized and subsequently released. While previous investigations hinted at the onset of three-dimensional (3D) protein structures forming within this tunnel, the precise mechanisms behind this phenomenon remained elusive. The authors of the study adopted a rigorous analytical approach, developing a model termed the Ribosome Environment Mimicking Model (REMM), which closely replicates both the dimensions and the chemical characteristics of the ribosome’s tunnel.
During their experiments, the researchers also compared the efficacy of REMM with a conventional carbon nanotube (CNT) model that emulates only the tunnel’s diameter but neglects its intrinsic chemical properties. This meticulous setup underscored the team’s commitment to differentiating between mere structural dimensions and the nuanced interplay of chemical interactions that occur during protein translation.
The Significance of Findings
The findings unveiled by this research hold critical implications for molecular biology. The simulations carried out using the REMM demonstrated a remarkable fidelity to experimentally observed protein structures when contrasted with those predicted by the CNT model. This accuracy reinforces the crucial role that chemical diversity within the ribosome tunnel plays in shaping protein conformation. As proteins begin to fold into their functional forms, understanding these interactions at a molecular level could lead to broader implications in biochemistry and related fields.
Moreover, the advantages of REMM don’t just stop at accuracy. The ongoing refinements to this model could significantly bolster our understanding of how proteins adopt their unique shapes as they pass through the ribosomal tunnel, potentially guiding future research in protein engineering and therapeutic applications.
This groundbreaking study underscores the necessity for innovations in modeling cellular processes, particularly in the context of protein synthesis. With the REMM as a robust tool, researchers are better positioned to explore the complexities of ribosome functionality and protein folding mechanisms. Continued advancements in this area may not only clarify our understanding of fundamental biological processes but also lead to transformative applications in medicine and biotechnology.
The exploration of the ribosome’s tunnel through advanced modeling techniques reveals an exciting frontier for scientific inquiry. As more knowledge surfaces, the possibilities to enhance our comprehension of cellular machinery and its implications for health and disease continue to expand, paving the way for a future where synthetic biology may mimic nature with ever-greater precision.

Leave a Reply