In the rapidly evolving field of battery technology, lithium and sodium metal anodes have emerged as key contenders in the quest for high-performance solid-state batteries. Solid-state batteries promise enhanced safety, energy density, and longevity, positioning them as a potential replacement for conventional lithium-ion batteries. However, the performance and stability of these batteries heavily hinge on the behavior of their anodes. Understanding the microstructure of lithium and sodium — particularly how they behave at the nano and microscopic levels — is crucial for the development of safer and more efficient energy storage systems.
Historically, the study of the microstructure of metals, including lithium and sodium, faced significant obstacles. Alkali metals exhibit extreme reactivity, forming thick layers of oxide or other reaction products upon exposure to environmental conditions. This reactivity obscures the underlying metallic structure, making traditional analysis methods ineffective. Researchers have struggled to image and characterize these metals, limiting advancements in anode design and the understanding of their electrochemical properties.
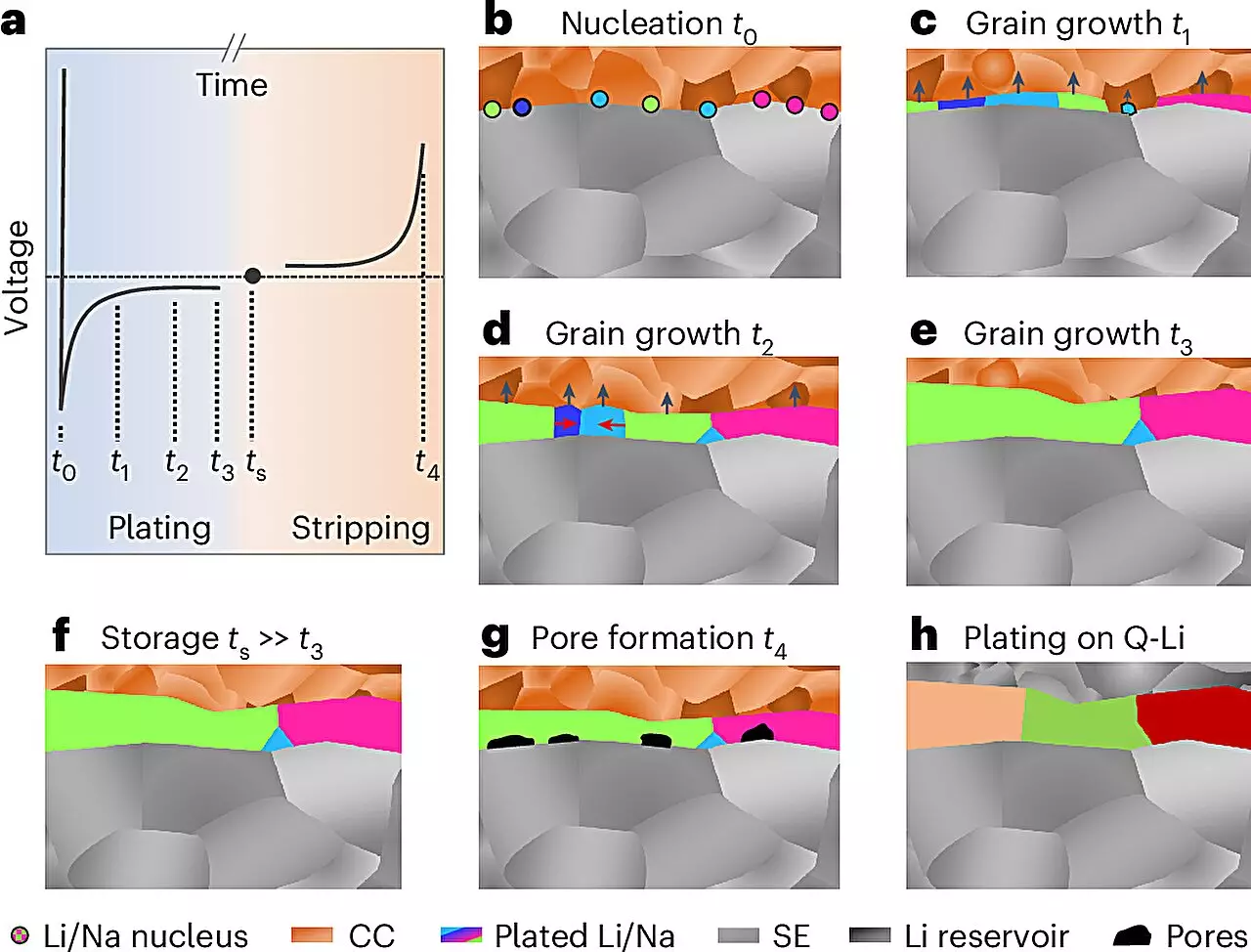
Recent advances made by a collaborative research team from Justus Liebig University Giessen (JLU), alongside partners from the University of California, Santa Barbara, and the University of Waterloo, mark a pivotal moment in this field. The researchers developed a novel methodology for analyzing electrochemically deposited lithium and sodium metals, successfully elucidating their microstructures for the first time. By performing the analyses at low temperatures and under controlled inert gas environments, the team overcame significant barriers to imaging these reactive metals.
The approach used electron backscatter diffraction, a sophisticated technique that allowed the researchers to map the internal structure of the metals with remarkable precision. “The grain size of the produced layers was surprising, and the insights revealed about the growth mechanisms are invaluable,” explains Prof. Dr. Jürgen Janek, the lead researcher from JLU. This insight is particularly crucial as it opens avenues for targeted manipulation of the microstructure to enhance the electrochemical performance and longevity of the batteries.
Insights into Growth Mechanisms and Their Implications
The study’s findings have broader implications for the design of future solid-state batteries. By understanding the microstructural properties of lithium and sodium, researchers can begin to identify ways to optimize the deposition processes, thereby mitigating issues such as pore formation and dendrite growth. Dendrites — tree-like structures that can develop during charging — pose significant risks, including the potential for short circuits. The ability to study the microstructure at this level offers a pathway to preventing these failures and improving overall battery safety.
Additionally, the work has implications for sodium-based batteries, which are quickly gaining attention as a cost-effective alternative to lithium batteries. As the team from JLU notes, their findings on sodium metal will contribute significantly to ongoing research efforts within the POLiS (Post Lithium Energy Storage) excellence cluster, which aims to explore sodium battery technologies and their potential applications.
The Path Forward for Solid-State Batteries
Despite the progress made, several challenges remain in utilizing lithium and sodium metal anodes in solid-state batteries. The inherent deformability of these metals during electrochemical cycling needs to be addressed. For effective battery performance, metal should ideally form only during initial charging, minimizing the risks associated with handling highly reactive alkali metal foils. This goal aligns with the broader industry push for batteries that not only deliver superior performance but also ensure a long operational life and enhanced safety measures.
As researchers continue to refine their understanding of metal anode microstructures, collaborations like those that produced this groundbreaking study will remain essential. The intricate interplay between materials science and electrochemical engineering will define the trajectory of battery technology, paving the way for new innovations.
The recent strides made in revealing the microstructure of lithium and sodium metal anodes represent a significant leap forward in solid-state battery research. As teams like JLU’s foster collaboration across international lines, the future of battery technology looks increasingly promising. The integration of detailed microstructural insights into the design of solid-state batteries could herald a new age of energy storage solutions that are not only powerful but also safe and durable. The momentum generated by these discoveries signals an exciting path forward for the development of advanced battery systems.

Leave a Reply