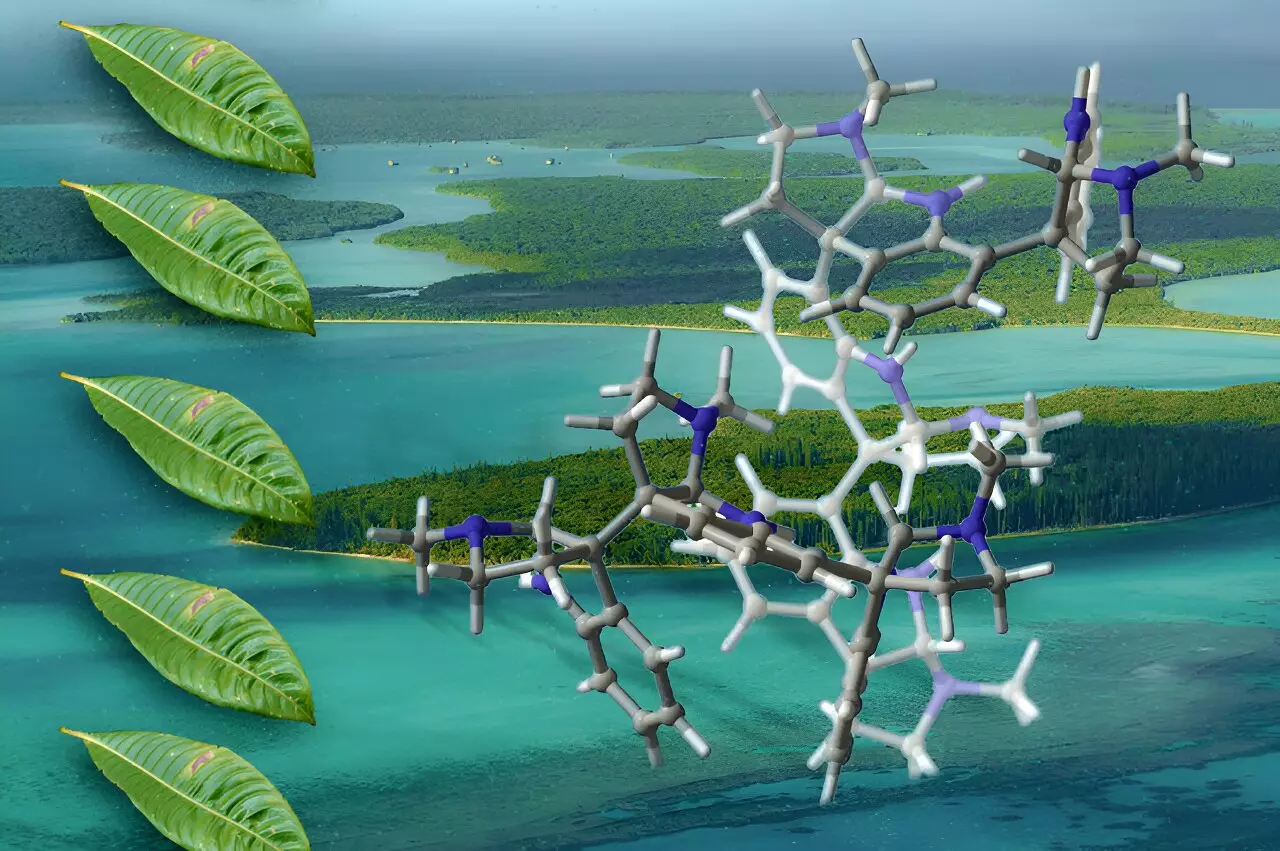
In the ever-evolving landscape of medicinal chemistry, the synthesis of complex molecules derived from natural sources is a frontier that holds immense promise. Among these are oligocyclotryptamines, intricate compounds discovered within select plant species that have demonstrated potential as antibiotics, analgesics, and cancer therapeutics. Recent work by chemists at the Massachusetts Institute of Technology (MIT) marks a significant advancement in the synthesis of these compounds, pushing the boundaries of what is not only possible in laboratory settings but also paving the way for groundbreaking studies in drug development.
Oligocyclotryptamines belong to a type of organic molecules known as alkaloids, which are primarily produced by plants and are characterized by their unique nitrogen-containing structures. These compounds consist of multiple tricyclic substructures known as cyclotryptamines, intricately fused through carbon-carbon bonds. This intricate architecture creates a challenge for synthetic chemists, primarily because only minute quantities of these naturally occurring oligocyclotryptamines can be isolated.
Given their structural complexity, oligosyclotryptamines represent a class of compounds that has attracted scientific interest since the 1950s, with years dedicated to understanding and characterizing smaller variants. However, earlier attempts to synthesize the larger versions—those adorned with six or seven fused rings—have been largely unsuccessful. This roadblock has delayed invaluable research into their pharmacological properties.
Innovative Synthetic Techniques: Diazene-Directed Assembly
The breakthrough by the MIT team relies on a novel methodology known as diazene-directed assembly, conceived by Mohammad Movassaghi and his lab. Traditional synthesis routes tend to falter when attempting to establish carbon-carbon bonds in densely substituted environments. The innovative approach developed by Movassaghi and lead author Tony Scott employs controlled radical chemistry to facilitate these crucial linkages.
The science underpinning this method involves manipulating carbon atoms into a radical state—characterized by the presence of an unpaired electron—creating highly reactive intermediates that can be guided through selective bonding. This control is achieved by tethering the carbon components with nitrogen atoms, utilizing light to trigger a reaction that liberates gaseous nitrogen and simultaneously enables the desired carbons to bond efficiently.
Movassaghi’s diazene-directed process not only simplifies the bond formation but also ensures the correct stereochemistry is maintained throughout the synthesis. The precision offered by this approach represents a significant leap in organic synthesis, as it allows chemists to tailor the construction of complex oligocyclotryptamines in a manner unprecedented in the field.
Implications for Drug Discovery and Development
One of the most enlightening aspects of this research is the potential impact on drug discovery. The ability to generate sufficient quantities of oligocyclotryptamines could lead to rigorous studies that explore their therapeutic properties, which have, until now, been hindered by the lack of availability of these compounds. Movassaghi expressed optimism about the implications of this work, stressing that reliable access to these compounds will enable scientists to conduct extensive investigations into their pharmacological effects.
Moreover, the synthetic methodology can be adapted to create new variations of oligocyclotryptamines, possibly enhancing their medicinal properties. The researchers aim to innovate further by assembling new derivatives that could outperform the naturally occurring versions, thus broadening the pharmacological arsenal available to modern medicine.
The reverberations of this research extend beyond MIT’s laboratory. Global chemistry experts, such as Seth Herzon from Yale University, have praised the work as revolutionary, highlighting its role as a “tour de force in organic synthesis.” The synthesis of oligocyclotryptamines is an exciting milestone, but it is essential to recognize that this is merely a foundation for future explorations.
As the scientific community rallies around these advances, collaborative efforts will be crucial in unlocking the full therapeutic potential of oligocyclotryptamines. Researchers will likely seek to build on Movassaghi’s methodology, dissecting the underlying mechanisms of these compounds while continuing to innovate on synthesis techniques.
The recent achievements of MIT chemists in synthesizing oligocyclotryptamines stand as a testament to the power of innovative thinking and interdisciplinary collaboration in advancing medicinal chemistry. With the promise of enhanced drug discovery and development, this breakthrough offers hope not just for future therapeutic interventions, but also for a deeper understanding of how complex natural products can be harnessed for human health. As researchers continue to innovate and explore the depths of molecular synthesis, the implications for medicine are poised to expand exponentially.

Leave a Reply