Adenosine triphosphate, commonly known as ATP, serves as the primary energy currency in biological systems. This crucial molecule fuels nearly all cellular processes, including muscle contraction, transportation of substances across membranes, and even the replication of genetic material. As such, understanding the mechanisms that govern ATP synthesis is a focal point of biochemical research. Recent findings by an international research team have illuminated an essential aspect of this process, revealing how a common yet vital atom shapes the chemistry of ATP production.
Magnesium ions are well-known for their catalytic functions in various biochemical reactions. They serve as cofactors for many enzymes, facilitating crucial activities in metabolic pathways. Among these enzymes is the adenylate kinase, which catalyzes the conversion of adenosine diphosphate (ADP) and adenosine monophosphate (AMP) into ATP. While the electrostatic influence of magnesium in promoting these reactions has been recognized, the specific biochemistry underlying its role demanded deeper scrutiny. This is where the pioneering research led by Professor Magnus Wolf-Watz at Umeå University comes into play.
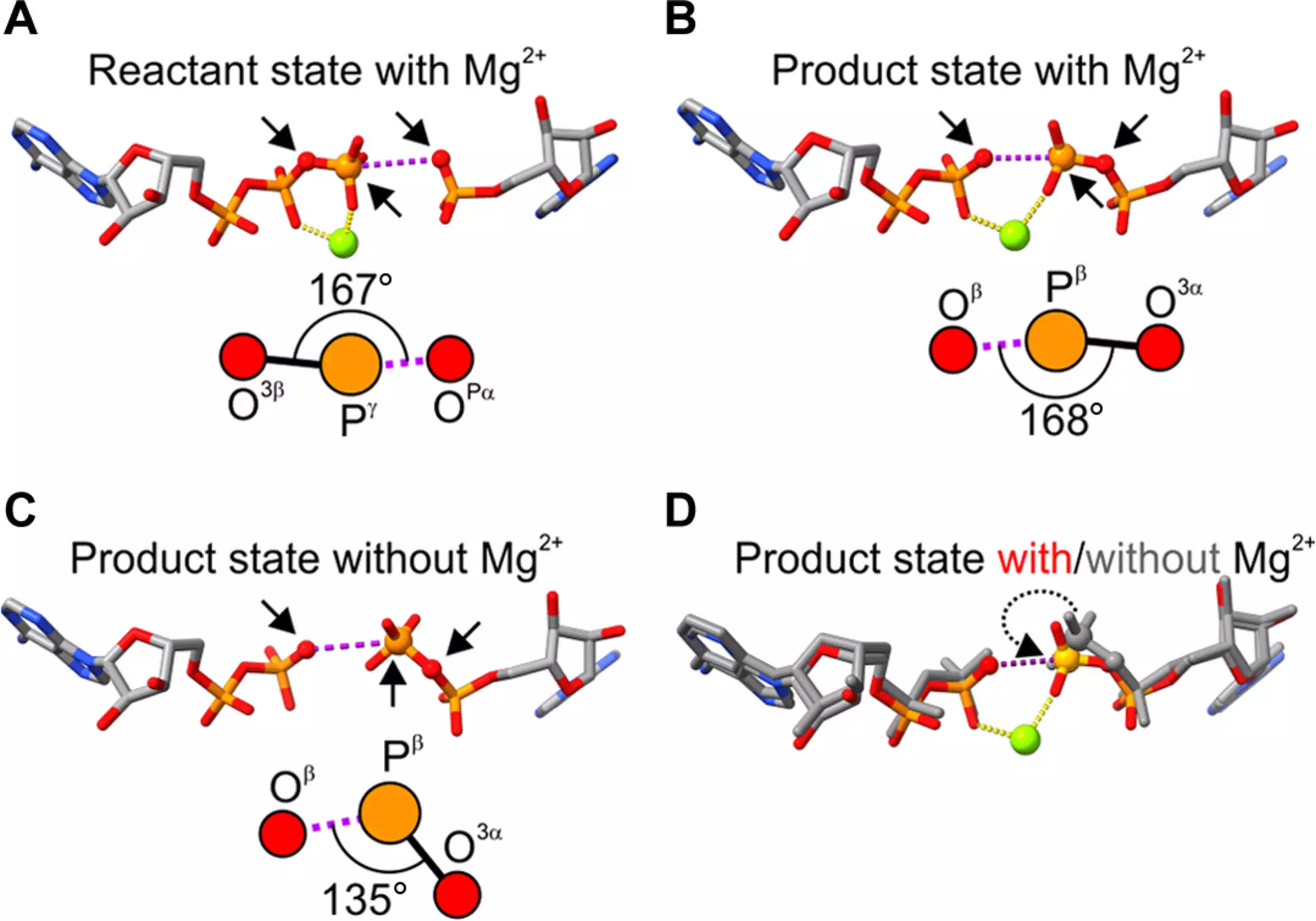
The team’s investigative endeavor not only reaffirmed magnesium’s catalytic prowess but also unveiled previously unnoticed complexities in its interaction with ATP precursors. Employing advanced crystallographic techniques, the researchers demonstrated that the alignment of ADP and AMP molecules is critical for optimal ATP formation. Their findings pointed to a remarkable scenario: when a magnesium atom shifts its angle in relation to these building blocks, the enzymatic reaction accelerates dramatically.
This “astonishing” finding, as described by Professor Wolf-Watz, indicates that tiny variations in molecular geometry can yield significant catalytic consequences. It shifts the paradigm of how we understand enzyme performance, establishing a link between the physical structure of molecules and their functional capabilities in biochemical reactions.
The researchers’ work didn’t stop with experimental observations. By collaborating with computational chemists, they were able to model the structural implications of the angles between the interacting molecules. Kwangho Nam from the University of Texas at Arlington played a crucial role in this computational analysis, establishing a crucial correlation between the magnesium-induced geometrical changes and the broader structural shifts within the enzyme itself. This linkage marks a significant advancement, offering critical insights into the foundational principles guiding enzymatic efficiency and selectivity.
The implications of these findings extend far beyond mere academic curiosity. Understanding how magnesium influences ATP production could yield insights applicable to various fields, including cellular biology, biochemistry, and even pharmacology. Given ATP’s central role in various biological processes, disruptions in its synthesis can have cascading effects on cellular health, potentially leading to conditions such as metabolic disorders.
As research continues to unearth the multifaceted roles of metal ions in cellular processes, the potential for therapeutic innovation based on these principles grows increasingly plausible. By understanding how to manipulate the enzymatic processes that rely on ATP, researchers could develop novel strategies to address diseases that stem from metabolic dysfunctions or inefficient energy usage.
The discoveries surrounding magnesium’s pivotal role in ATP synthesis are a testament to the sophisticated intricacies of biochemical interactions within living organisms. As scientists unravel these complexities piece by piece, potential new avenues for understanding and treating various ailments will become apparent. With further research and exploration, the pathways illuminated by these findings could inspire groundbreaking advancements in both basic science and clinical applications, ensuring a brighter future in our quest for knowledge and health.

Leave a Reply