In a groundbreaking study published in Nature Communications, researchers from the Interface Science Department at the Fritz Haber Institute have made a significant advancement in the fight against climate change. Their study introduces a new method for understanding the mechanisms of carbon dioxide (CO2) re-utilization, leading to the production of fuels and chemicals. This breakthrough in catalyst control opens up a world of possibilities for the optimization of catalytic processes driven by renewable electricity.
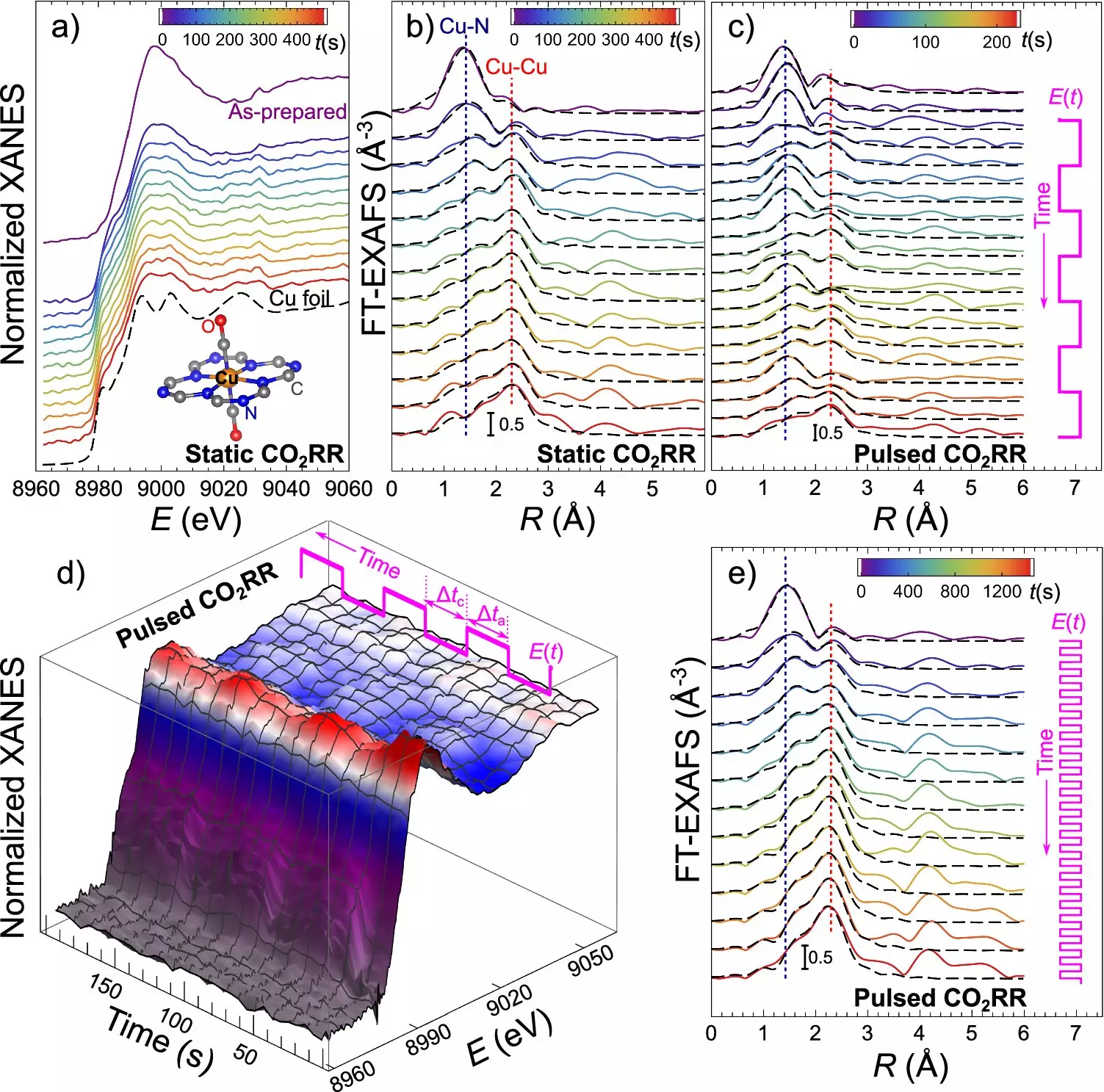
At the core of this discovery lies the unique properties of catalysts composed of ultradispersed copper and nitrogen atoms incorporated into carbon. These catalysts exhibit the ability to dynamically change from single atoms to small clusters and nanoparticles during the electrocatalytic CO2 reduction (CO2RR) process. This reversible transformation of the catalyst structure provides a key for steering the outcome of the CO2RR process, as the product selectivity is strongly influenced by the catalyst’s state.
One of the key challenges in scaling up CO2RR technology for practical use has been the broad distribution of different reaction products, making it difficult to produce specific chemicals and fuels efficiently. However, this research offers a method to precisely control the distribution of CO2RR products by manipulating the catalyst’s structure. By using alternating electrical pulses, researchers can control the size and structure of the catalyst particles, ultimately influencing the production of specific reaction products.
Different forms of the catalyst are better suited for producing specific CO2RR products. For example, single copper atoms are efficient for hydrogen production, while small clusters favor methane and larger nanoparticles are best for ethylene production. By varying the duration of electrical pulses, researchers can steer the sizes of formed nanoparticles, allowing for precise control over the catalyst’s state and product selectivity.
To monitor and adjust the catalyst’s transformation in real-time, the research team utilized operando quick X-ray absorption spectroscopy. This advanced technique provides sub-second time resolution, allowing scientists to observe the catalyst’s structural changes during the reaction. By ensuring optimal conditions for desired CO2RR products, this method sets the stage for future advancements in the field of catalysis and renewable energy technologies.
This study not only deepens our understanding of catalyst behavior and structural transformations during operation but also sheds light on the potential applications of CO2 reduction in greenhouse gas mitigation and the production of green chemicals and fuels. By controlling the catalyst’s structure, researchers have unlocked new pathways for technological advancements in carbon dioxide utilization, marking a significant stride in scientific inquiry and innovation.
Leave a Reply