In recent years, the environmental crisis has been exacerbated by the presence of micropollutants in our waterways. Substances like pesticides and industrial chemicals have permeated our ecosystems, posing risks to both human health and biodiversity. Traditional methods of treating wastewater have struggled to effectively break down these persistent compounds, necessitating innovative approaches to tackle the issue head-on. One promising solution lies in photocatalysis—a process that utilizes light to accelerate chemical reactions—combined with advanced nanomaterials.
Photocatalysis: A Game-Changer in Pollutant Removal
Photocatalysis operates on the premise that certain semiconducting materials can harness sunlight to drive chemical reactions that degrade harmful pollutants into less toxic byproducts. Among the most frequently used materials in photocatalysis is titanium dioxide (TiO2), renowned for its stability and efficiency. However, researchers have continuously sought methods to enhance TiO2’s effectiveness, particularly by using metal nanoparticles like gold as co-catalysts. These enhancements could potentially broaden the reach of photocatalytic activity, allowing for more effective treatment of toxic substances.
Visualizing the Process: A Breakthrough Imaging Technique
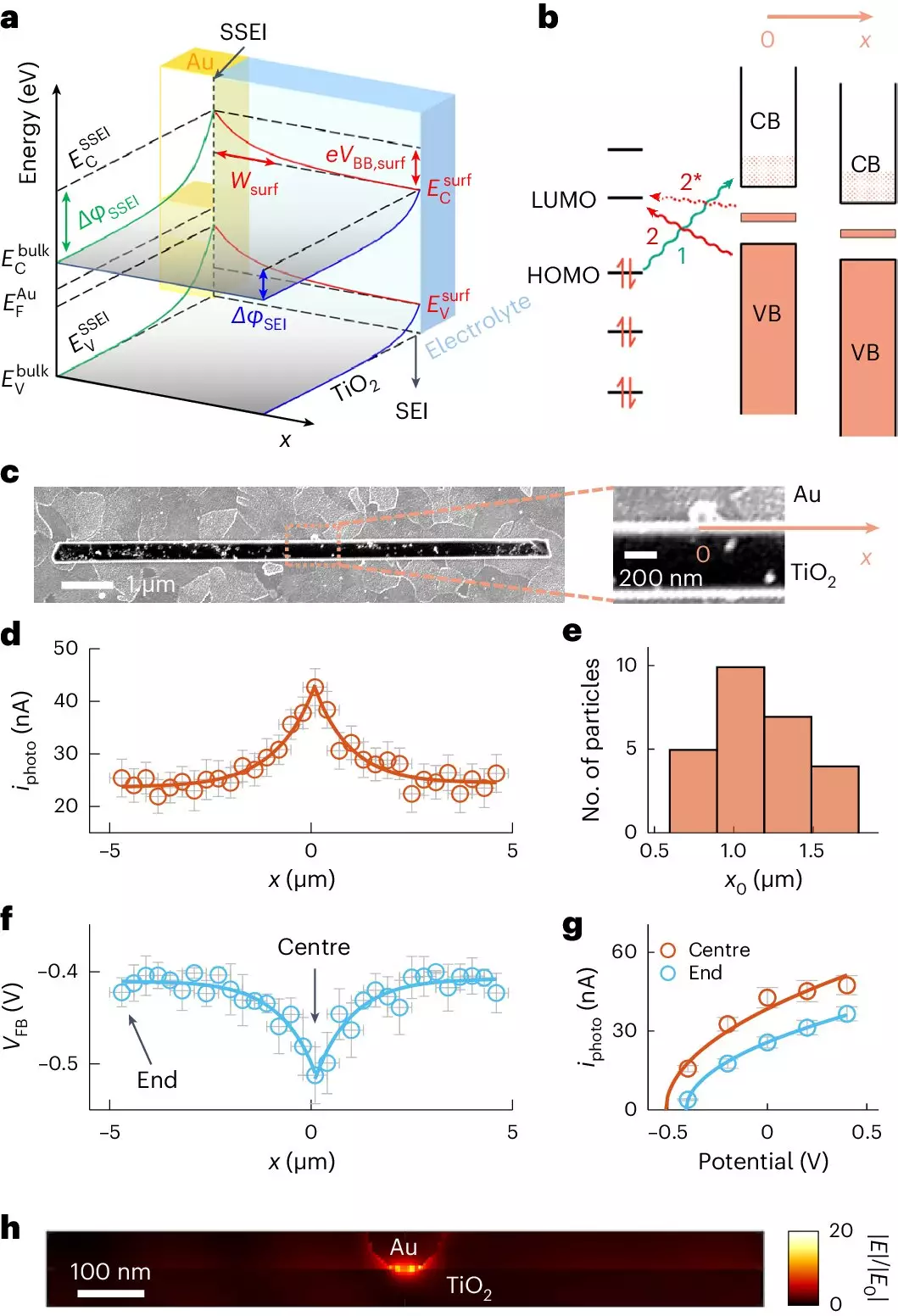
A groundbreaking study led by a team from Cornell University has made significant strides in understanding how gold nanoparticles impact the behavior of TiO2 in photocatalytic applications. By adopting a novel imaging approach termed adCOMPEITS (Adsorption-based COMPetition Enabled Imaging Technique with Super-resolution), the researchers were able to visualize how substances adhere to the surface of the TiO2. This method involves monitoring the competition between fluorescent probe molecules and non-fluorescent micropollutants, enabling scientists to measure adsorption at a level of precision previously unattainable.
The findings revealed a strikingly broad enhancement of adsorption rates around gold nanoparticles—extending as much as ten times farther than previous models had suggested. This discovery could catalyze a new wave of advancements in the field of environmental remediation.
The Science Behind the Enhancement
At the core of the gold-enhanced photocatalytic reaction lies a phenomenon known as surface band bending. The gold nanoparticles influence the electronic properties of the TiO2, bending energy bands in a way that optimizes the adsorption of pollutants. This mechanism allows the transformation of solar energy into chemical energy to occur not just locally around the nanoparticle but over a considerable distance.
This long-range effect is particularly beneficial for practical applications, as it suggests that even a small quantity of gold can significantly enhance the efficiency of TiO2. The implications are profound: enhanced performance could lead to lower operational costs and improved treatment outcomes, making the technology more viable for widespread use in wastewater management.
Implications for Environmental Remediation
The research opens up exciting possibilities for how we approach micropollutant removal in wastewater. With an increasing array of harmful contaminants entering our water systems, the need for effective, economically feasible solutions has never been more urgent. The combination of photocatalysis with innovative imaging techniques not only furthers our understanding of the underlying science but also brings us closer to practical applications that can remediate polluted water on a large scale.
Additionally, the insights gained from this research could extend beyond pollutant removal. The enhanced adsorption mechanism could find applications in areas such as chemical sensing or the development of more efficient dye-sensitized solar cells, further amplifying the benefits of this research beyond mere environmental cleanup.
Future Directions and Broader Applications
The research team envisions that the principles identified in this study will be applicable in various domains, beyond just photocatalysis. The ability to leverage tiny quantities of gold and achieve significant enhancements in several chemical processes could revolutionize how we think about material use and efficiency in both environmental and energy applications. As further exploration of this technology unfolds, the synergy between nanotechnology and catalysis is poised to play a pivotal role in shaping a more sustainable future.
The incorporation of gold nanoparticles into photocatalytic systems represents a stunning illustration of how science continuously evolves to address real-world issues. By enhancing our understanding of these processes, researchers are paving the way for innovative strategies to combat pollution while promoting greener technologies for future generations.

Leave a Reply