Polymers are ubiquitous in both nature and industry, often likened to trains made up of numerous cars, where each car represents a monomer, the fundamental building block of the polymer. The connections between these cars can be thought of as the chemical bonds that link monomers. This analogy highlights the complex structure of polymers, which are vital in a diverse range of applications, spanning from drug delivery systems to the construction industry. However, the properties and functionalities of these polymers are frequently hampered by the fact that they predominantly rely on a limited variety of chemically similar monomer types, which can restrict their application in novel technologies. This brought attention to the pressing need for innovative methodologies to synthesize new monomers, with the potential to enhance the capabilities of polymer-based systems.
Recent research led by Scripps Research chemists, in collaboration with polymer scientists from the Georgia Institute of Technology and the University of Pittsburgh, has made significant strides in overcoming these limitations. By devising a novel reaction that employs nickel as a catalyst, the team has successfully facilitated the controlled creation of unique monomers. The findings, published in Nature Synthesis, reveal that these newly synthesized monomers can lead to polymers with enhanced and modifiable properties, essential for fields such as drug delivery, energy storage, and advanced microelectronics.
The innovative approach hinges on the ability to manipulate the existing chemical structure of a molecule, ultimately allowing researchers to introduce distinct functional groups. According to Dr. Keary Engle, a prominent figure in this research, utilizing earth-abundant metal catalysts like nickel not only expands the variety and complexity of materials achievable but also allows scientists to venture into previously unexplored territory regarding structural and functional diversity in polymer chemistry.
A pivotal aspect of this research is the collaborative effort among researchers from different institutions. This interdisciplinary approach provided a platform for evaluating whether the strategies employed for small molecule synthesis could be effectively translated to the realm of polymer synthesis. Anne Ravn, co-first author of the paper and postdoctoral researcher in Engle’s lab, emphasized that modifying the fundamental chemistry of the monomer building blocks allows for innovative applications in constructing the larger macromolecular structures needed for various technological advancements.
The multi-institutional team involved numerous contributors, each bringing unique perspectives and expertise necessary for the successful development of these new chemical reactions. The culmination of their work resulted in a method that not only produces novel monomers but also enables the assembly of these into polymers with unprecedented structural attributes.
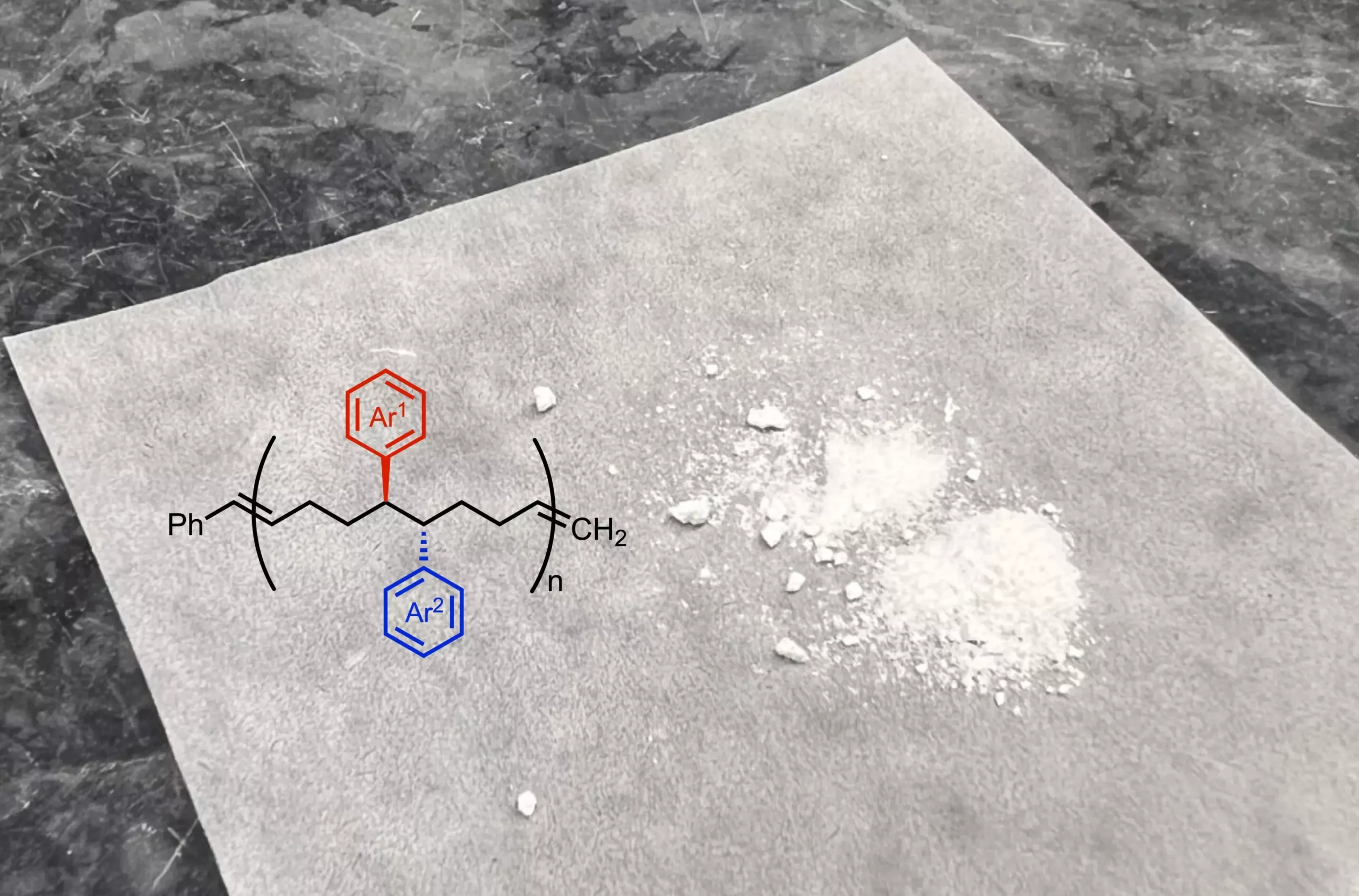
The crux of the team’s breakthrough involves a nickel-catalyzed reaction that effectively alters starting molecules by incorporating two new functional groups. These small side chains change the characteristics of the resulting polymers, influencing their properties in terms of flexibility, elasticity, and solubility. Since traditional commercial polymers often maintain a two-carbon spacing between functional groups, the introduction of additional functional groups at closer intervals creates a profound impact on the material’s properties. This technique allows for a “decoration” of the polymer backbone in ways previously unattainable.
Moving forward, the research team is keen to investigate how incorporating varying functional groups into the monomer structures might further modify the resultant material’s properties. Ravn highlighted that this method permits a greater flexibility in exploring different functionalities within the equivalent polymer constructions.
An essential advantage of this new synthesis method lies in its potential for environmental sustainability. Nickel’s abundance as compared to other metal catalysts used in chemical reactions positions this method as not only economically viable but also more sustainable. The research team is actively exploring avenues to further enhance the ecological footprint of their product through the degradation of long polymers into their original building blocks for reusability.
This dual focus on functionality and sustainability aligns with the broader goals of modern chemistry to develop materials that are both effective and environmentally responsible. By reducing the waste inherent in polymer production and promoting circular economic principles, the findings from this study could pave the way for future advancements in material science.
The innovative strategies developed to create unique monomers signal a significant step forward in polymer chemistry, offering a blend of versatility and sustainability that can transform applications across numerous industries. The collaborative efforts of the research teams underscore the importance of interdisciplinary approaches in addressing modern challenges, potentially leading to a new era of advanced materials with boundless applications.

Leave a Reply