Recent advancements in analytical chemistry have heralded a new era for the detection and analysis of nuclear materials. Researchers at Oak Ridge National Laboratory (ORNL) have successfully combined two sophisticated techniques to simultaneously identify fluorine and various isotopes of uranium in individual particles. This milestone not only enhances our understanding of nuclear substances but also provides the International Atomic Energy Agency (IAEA) with valuable tools for nonproliferation efforts.
Fluorine plays a pivotal role in converting uranium into a chemically suitable form for enrichment processes. By being able to detect both elements at the same time, scientists can glean essential insights into the intended use of nuclear materials, which is critical for ensuring global security. The ability to quickly analyze these elements is paramount for regulatory agencies tasked with monitoring and inspecting nuclear materials worldwide.
Isotopes are distinct variations of a chemical element that have the same number of protons but differ in the amount of neutrons. This variance leads to differences in their nuclear properties, making isotopes crucial for dating materials and understanding various chemical processes. The ability to determine isotopic ratios in single particles has long been a time-consuming endeavor; however, ORNL’s innovative approach significantly accelerates this process. Benjamin Manard, the leading researcher on the project, emphasized the urgency and importance of rapid particle analysis for both fluorine and uranium isotopes.
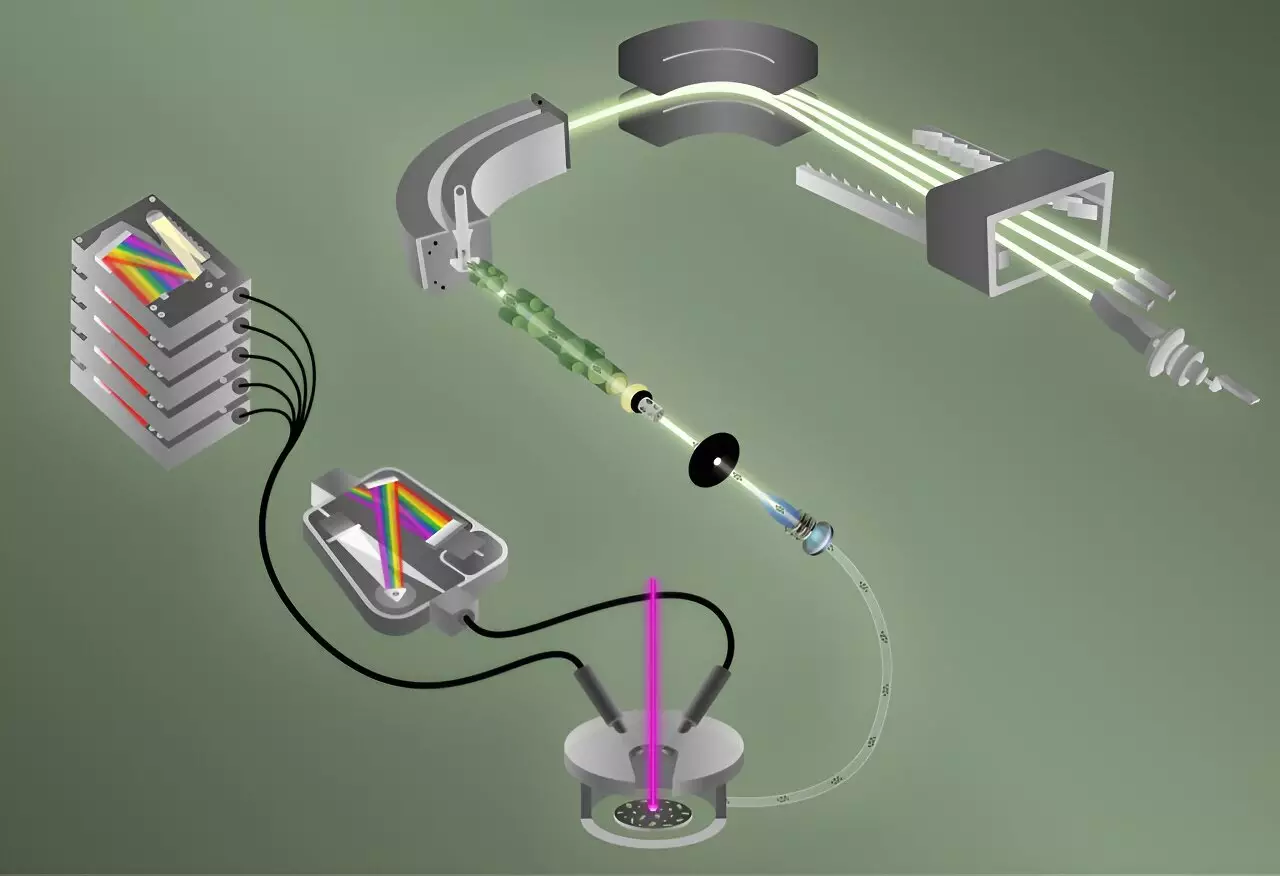
The researchers cleverly employed two complementary methods to analyze particles, each no larger than a red blood cell, within mere minutes. The initial technique utilized is laser-induced breakdown spectroscopy (LIBS), which is known for its rapid and sensitive detection of elemental fluorine. This method involves vaporizing a sample to create a plasma, which emits light as it cools. Different elements within the sample produce distinct emission wavelengths—like colorful bursts in fireworks—allowing for efficient characterization.
Simultaneously, the scientists introduced helium gas to sweep the plasma into a mass spectrometer, where an advanced technique known as laser ablation multicollector inductively coupled plasma mass spectrometry (ICP-MS) could analyze uranium isotopes. This second technique involves heating the plasma to extreme temperatures, surpassing those found on the surface of the sun, to produce positive ions necessary for the measurement process.
The simultaneous detection of fluorine and uranium within individual particles holds significant implications for nuclear material analysis. Brian Ticknor, a co-author of the research, pointed out that understanding the balance between these two elements can provide insight into the origins of a particle and the processes that produced it. Moreover, beyond national security concerns, the methodology developed has far-reaching applications in various sectors, such as next-generation battery manufacturing and environmental science focusing on the behavior of microplastics.
Historically, combining LIBS and ICP-MS for simultaneous measurements posed challenges due to their contrasting demands; LIBS excels at detecting nonmetals like fluorine, whereas ICP-MS is primarily effective for positively charged ions common among metals. Therefore, the integration of these two techniques required innovative thinking and deep collaboration within ORNL’s multi-disciplinary team. The complexity of these tasks highlights the importance of cross-departmental research in advancing scientific frontiers.
Looking ahead, Manard and his colleagues are eager to expand their methods to include the analysis of other complex compounds, including those containing chlorine, which shares resemblances with fluorine in terms of its electronegative characteristics. Through continued development, the team aims to enhance particle analysis speed, aspiring to characterize thousands of particles within just a 24-hour period.
Additionally, recent collaborative efforts have seen applications of this technology reach beyond nuclear materials. In a fascinating study, researchers examined shark teeth to understand historical environmental conditions. Such interdisciplinary applications of the combined techniques underscore their versatility and potential to enrich knowledge across various scientific domains.
The groundbreaking work at ORNL signifies a pivotal advancement in the realm of nuclear material analysis. By uniting two powerful analytical strategies, scientists have opened new avenues for the forensic examination of substances that are crucial for understanding both the past and present implications of nuclear chemistry. As research continues, the implications of this innovation are bound to resonate across myriad scientific disciplines, heralding a new frontier in analytical capabilities.

Leave a Reply