The realm of material science is ever-evolving, particularly with emerging classes of compounds that offer transformative potentials. Among these, Ruddlesden-Popper compounds stand out due to their unique layered structures, fostering exceptional attributes suitable for applications ranging from superconductors to efficient catalysts, and even promising technologies in photovoltaics. Historically, this class primarily included halides and oxides. However, the advent of Ruddlesden-Popper nitrides marks an extraordinary milestone, paving the way for an array of novel applications and scientific explorations.
The Challenge of Nitrogen Synthesis
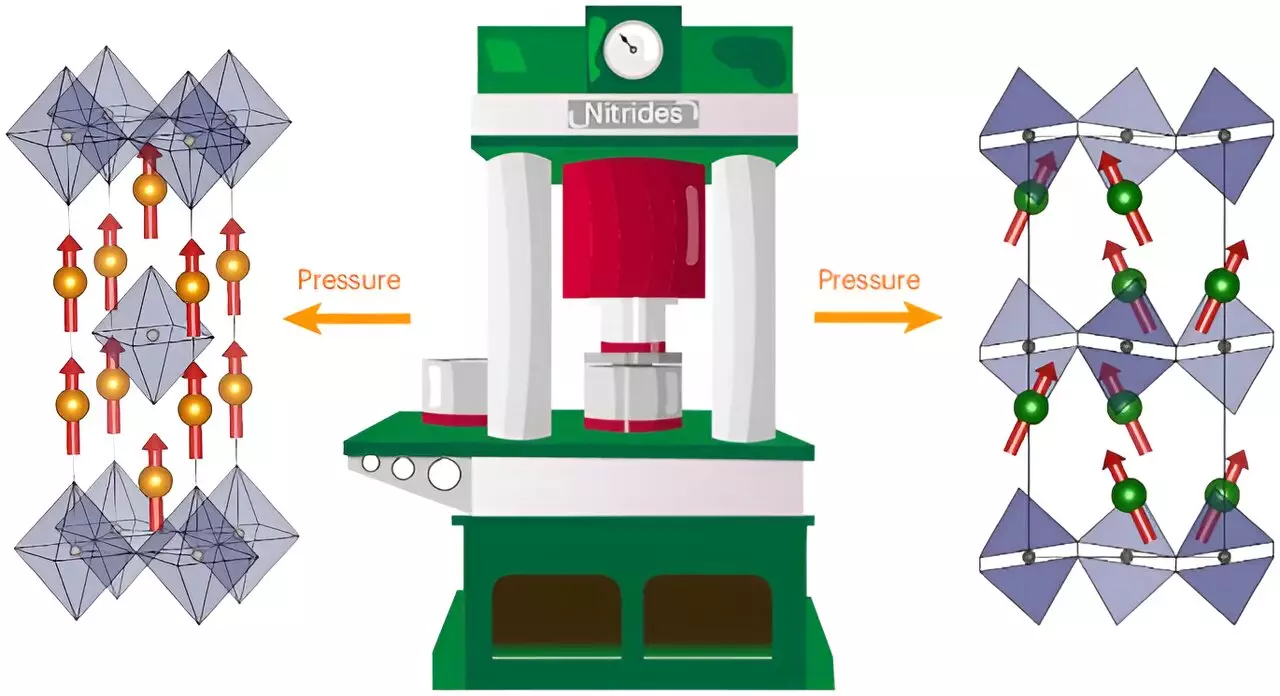
Despite initial scientific optimism surrounding the potential of Ruddlesden-Popper nitrides, the actual synthesis remained a daunting challenge due to the stability of nitrogen molecules (N2) and the low electron affinity of nitrogen itself. These properties rendered the traditional methods ineffective for creating the nitrides. It wasn’t until a team led by Dr. Simon Kloß from the Department of Chemistry at LMU that a breakthrough synthesis pathway was developed, opening new doors in material chemistry.
Using extreme conditions—including high pressures of 8 gigapascals—this innovative approach involved compressing samples with an active nitrogen source, like sodium azide. This drastic alteration to the synthesis environment symbolizes the essence of modern chemistry: necessitating creativity and resilience in overcoming fundamental scientific hurdles.
The Implications: Discovering New Compounds
The research detailed in the journal *Nature Chemistry* unveiled three novel compounds: cerium-tantalum nitride (Ce2TaN4) and praseodymium- and neodymium-rhenium nitrides (Ln2ReN4). These compounds are not just mere additions to the existing catalog of materials; they present a robust variety of structural, electronic, and magnetic properties. For instance, the neodymium nitride exhibits hard ferromagnetic characteristics with irreversible magnetic behavior, a prospect that could redefine applications in data storage and magnetic technologies.
On the other hand, the cerium-tantalum compound functions not only as a semiconductor but also as a potential ferroelectric material, facilitating energy conversion processes. These diverse functional properties suggest that Ruddlesden-Popper nitrides could be pivotal in developing next-generation devices that prioritize efficiency and sustainability.
Potential Future Applications
The implications of this research extend far beyond academic curiosity. The ability to systematically explore Ruddlesden-Popper nitrides opens a landscape brimming with possibilities. Enhanced superconductors, cutting-edge photovoltaic materials, and catalytically active compounds could emerge as practical technologies, transforming the energy landscape. Dr. Kloß’s synthesis strategy could potentially serve as a blueprint for future investigations into related compounds, fostering a richer understanding of their functional behaviors and leading to practical innovations.
As we stand on the brink of this exciting frontier, one cannot overlook the profound impact such advancements could have on various industries. A new generation of materials engineered for efficiency, performance, and sustainability might soon be within our reach, thanks to the ingenuity of material scientists navigating this fascinating territory. The exploration of Ruddlesden-Popper nitrides is thus not only a scientific achievement but a beacon of hope for future technological advancements.
Leave a Reply