The pharmaceutical industry faces a monumental challenge: developing a new drug. This Herculean task often stretches across years and demands financial investments that can easily reach into the millions. Alarmingly, over 90% of drug candidates do not survive the rigorous trials they must undergo to prove their safety and efficacy. The tragic irony is that many of these failures occur despite best efforts, largely due to safety concerns that arise when these compounds interact with human biology.
These issues in drug development are not merely statistics—they represent lives affected by unmet medical needs and inefficient resource allocation. The financial strain on companies, especially smaller biotechs, cannot be understated. Innovations that expedite the drug discovery process are desperately needed, and this is where advancements in artificial intelligence (AI) come into play.
AI Models: A Proactive Approach
Researchers from the Broad Institute of MIT and Harvard are pioneering the use of AI to influence drug development positively. By training predictive machine learning models, they aim to assess the potential biological effects of compounds before they are tested in organisms. This preemptive strike against drug toxicity can significantly streamline the pathway from initial conception to clinical trials.
The work led by Srijit Seal includes identifying distinguishing chemical and structural features in drugs that are correlated with potential toxic effects. By honing in on various biomarkers—such as general cellular health and the functions of vital organs like the heart and liver—these models can prioritize drug candidates that are less likely to cause adverse effects, significantly narrowing the pool that requires extensive laboratory testing.
Real-World Implications and FDA Collaboration
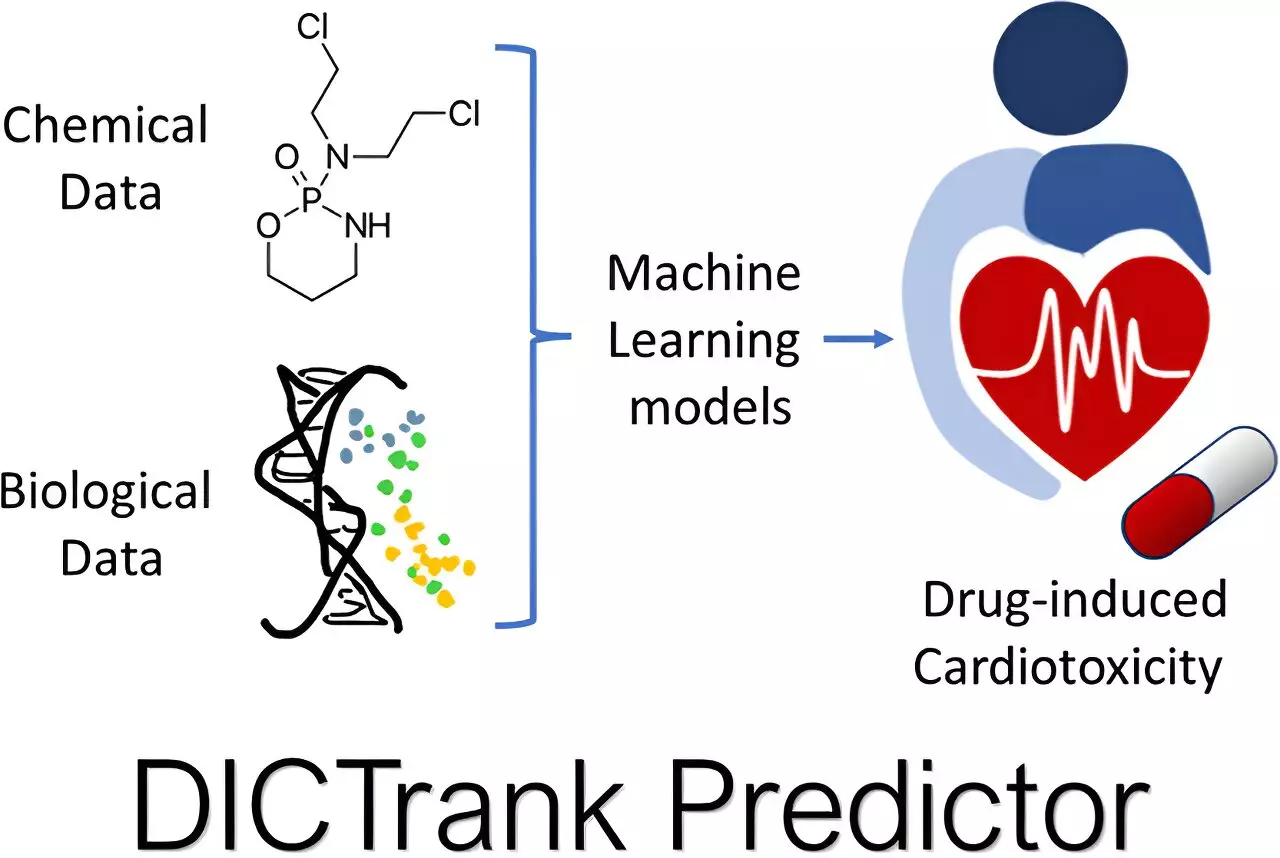
The FDA has underscored the importance of understanding drug toxicity through curated lists detailing which medications pose risks to heart and liver function. Seal utilized these datasets as the foundation for training his machine learning models, developed specifically for predicting drug-induced cardiotoxicity and liver injury.
One of the standout innovations, known as the DICTrank Predictor, represents the first instance of a predictive model assessing the FDA’s DICT ranking list. Given that similar compounds can yield vastly different responses across species (animal studies versus humans), the challenge of accurately predicting toxicity has been formidable. However, DILIPredictor has made remarkable strides, capable of identifying safe compounds for human use even when they exhibit toxicity in animal models. This kind of predictive power promises to save significant time and resources in the drug development pipeline.
Pushing the Envelope of Pharmacokinetics
As crucial as toxicity is, another critical facet of drug discovery lies in understanding pharmacokinetics—how a drug is absorbed, transported, metabolized, and cleared by the body. Early identification of these parameters can help researchers avoid wasting efforts on compounds that will fail due to ineffective pharmacokinetic profiles.
Enter the predictive pharmacokinetic modeling tool. Seal and his team are developing this innovative platform to help researchers loop back to the design phase, ensuring that the drug candidates under investigation uphold the required efficacy while minimizing potential toxic outcomes. “Machine learning in pharmacokinetics is becoming popular,” Seal states, emphasizing the rapid evolution of this methodology in the realm of drug design.
Deep Learning and Cellular Health: Bridging Complexities
Despite the predictive capabilities of these AI models, understanding the intricate biological mechanisms influencing drug actions remains an area of active discourse. This is where BioMorph, a deep learning model also developed by Seal, comes into play. It integrates imaging data from CellProfiler—an open-source tool for analyzing cell morphology—with traditional metrics of cell health, such as proliferation rates.
BioMorph adds a critical layer of interpretation, enabling scientists to assess the biological implications of compounds based on image data. Utilizing two datasets in training, the model enhances its accuracy in correlating specific compounds with the resultant effects on cellular features. This not only aids researchers in identifying potential hazards early in the process but also facilitates deeper biological insights that may guide future innovations.
The intricate interplay of drug discovery, AI technology, and biological understanding signifies that the industry is on the brink of a transformative leap. Armed with advanced tools and comprehensive algorithms, researchers may finally develop the means to transform drug successes into a more frequent reality, thereby ushering in a new era in medication safety and efficacy. The amalgamation of AI and pharmacology holds tremendous promise, potentially leading us closer to more effective therapies with minimized risk— a victory not only for the industry but for patients worldwide.

Leave a Reply