Cryopreservation is an essential technique in modern medicine, underpinning the storage and transportation of various biological materials, including vaccines, blood products, and fertility treatments. Essentially, the process involves freezing these materials at extremely low temperatures to preserve their cellular structure and efficacy. However, the challenge has long persisted that freezing and thawing can lead to ice crystal formation, a significant barrier that can render biological products ineffective. The traditional approach to discovering effective cryoprotectants—substances that inhibit ice formation during freezing—has been predominantly reliant on resource-intensive experimental methods. The pioneering research conducted by scientists at the University of Warwick and the University of Manchester has introduced a sophisticated computational model that promises to change the landscape of cryopreservation.
Published in *Nature Communications*, this breakthrough develops a computational framework that accelerates the identification and testing of potential cryoprotectants using advanced machine learning algorithms. Guided by the expertise of Professor Gabriele Sosso from the University of Warwick, the research aims to harness a synergy between machine learning and traditional molecular simulations to enhance the cryopreservation process. By leveraging a data-driven approach, the team can investigate a vast library of chemical compounds rapidly, which contrasts sharply with preceding methodologies that relied heavily on trial and error, making the process exceedingly slow and often costly.
Prof. Sosso asserts the importance of understanding that machine learning, while powerful, is not an all-encompassing answer to every scientific dilemma. Instead, it functions as a valuable tool that, in concert with rigorous experimental work, can lead to profound advancements. This collaborative approach fosters innovative thinking and equips researchers with the ability to make quicker, more informed decisions about which compounds to test in laboratories.
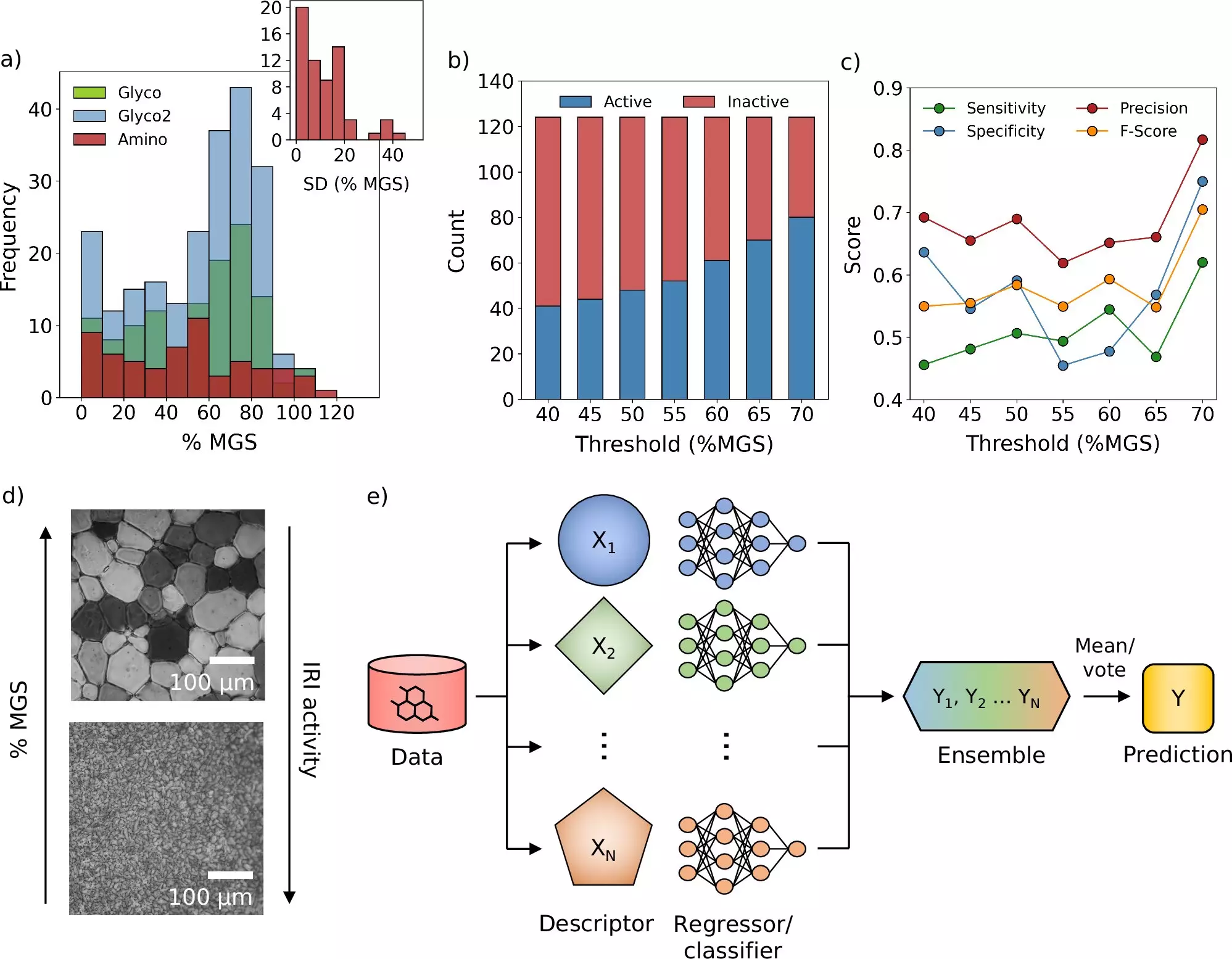
The research has yielded promising findings, specifically identifying a novel molecule capable of inhibiting ice crystal growth during the freezing process. This discovery stands as a monumental advancement, as current cryoprotectants succeed in safeguarding cells but do not effectively prevent ice formation. Dr. Matt Warren, the doctoral candidate who played a significant role in steering the project, highlighted the transformative nature of this model, noting that it enables a more systematic and data-driven determination of cryoprotective effectiveness.
Moreover, the implications of this research extend beyond theoretical models, showcasing real-world applications. The research team conducted empirical studies with blood samples and discovered that the newly identified molecules could reduce the traditional quantity of cryoprotectants needed for blood preservation. This reduction not only enhances the efficiency of blood storage but also expedites the thawing process, thus allowing for more rapid transfusion—a factor that can be critical in emergency medical situations.
The collaborative efforts of Prof. Sosso’s team and Prof. Matthew Gibson’s group at the University of Manchester highlight the importance of interdisciplinary research. Prof. Gibson has dedicated over a decade to examining the unique anti-freeze capabilities of ice-binding proteins found in polar fish, developing innovative biomimetic molecules that emulate these natural phenomena. The intersection of these research paths has significantly expedited the discovery process, yielding results that, according to Gibson, would have been unlikely to surface through conventional methods.
This remarkable progression underlines the notion that scientific innovation often thrives at the confluence of diverse expertise and technology. By bringing together different realms of knowledge, including computational biology and experimental cryobiology, researchers are setting the stage for breakthroughs that can significantly transform medical practices related to storage and distribution of biological materials.
As research into cryoprotectants evolves, the potential for new applications burgeons. With the computational framework now at the researchers’ disposal, the door is wide open for not only identifying superior cryoprotectants but also repurposing existing compounds that can mitigate ice growth. Looking forward, this significant leap in methodology could lead to remarkable efficiency gains in the preservation and transport of medical materials that are essential for patient care and advancement in therapies.
The intersection of machine learning and experimental research marks a new chapter in the journey of cryopreservation technology, ultimately contributing to a future where medical treatments remain viable and effective, regardless of storage conditions. As trials continue, the impact of these findings are poised to resonate across the medical community, pioneering safer and more effective solutions to complex biological challenges.
Leave a Reply