The need for cleaner and more efficient methods in chemical production has never been more critical. As the world shifts toward sustainability, researchers are exploring innovative technologies that reduce environmental impact while improving energy efficiency. A recent groundbreaking study from Lawrence Livermore National Laboratory (LLNL) highlights an electrochemical method that significantly enhances the production processes of valuable chemical compounds, demonstrating a successful collaboration between scientists to advance green chemistry.
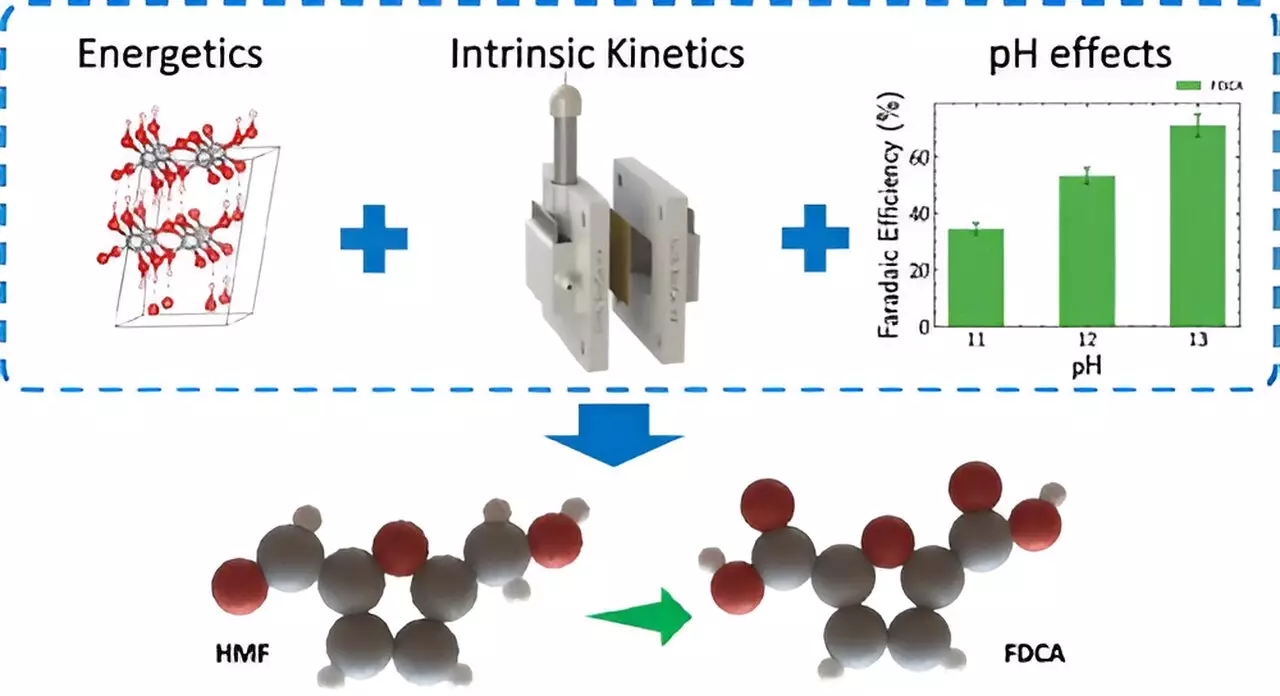
At the heart of this research lies the use of thin film nickel anodes, which provide a stable and consistent surface for studying electrochemical reactions. This approach mitigates the complexities introduced by porous or irregular structures, allowing for a more accurate analysis of catalyst behaviors. “By utilizing thin films, we can focus entirely on the intrinsic properties of the catalyst materials without additional variables interfering,” explained Aditya Prajapati, a postdoctoral researcher involved in the project. This clear-eyed focus on the catalyst is crucial for the understanding and enhancement of the chemical reactions involved.
Traditionally, electrolyzers, crucial for converting electrical energy into high-energy-density molecules, have faced challenges regarding energy efficiency, particularly linked to the oxygen evolution reaction occurring at the anode. The LLNL team innovatively proposed that replacing this reaction with biomass oxidation could potentially lower energy consumption by over 50%. This advancement not only highlights the importance of energy efficiency but also reflects a significant stride toward sustainable biomass conversion, which is pivotal in modern chemistry.
Transforming Biomass into Valuable Products
The electrochemical process introduced in this study focuses on transforming 5-hydroxymethylfurfural (HMF), a compound derived from biomass, into 2,5-Furandicarboxylic acid (FDCA). FDCA is a crucial building block for creating sustainable plastics, like the bio-based polymer PEF. This conversion exemplifies a shift from conventional high-temperature chemical methods, which often result in toxic byproducts, toward a cleaner, more sustainable alternative. Nitish Govindarajan, a contributing researcher, emphasized, “Our technique not only decreases reliance on petroleum but also minimizes carbon emissions.”
The implications of this research extend beyond immediate efficiency gains. By utilizing renewable biomass as a primary feedstock, the developed method inherently supports the principles of a circular economy. This approach reduces dependence on finite fossil fuels and fosters a transformation in how we perceive chemical production and its relationship with environmental sustainability. Importantly, when paired with renewable energy sources, this electrochemical process has the potential to achieve a zero-carbon footprint.
As this study demonstrates, the integration of electrochemical techniques and renewable resources can lead to significant advancements in sustainable chemical production. With researchers from LLNL and other institutions such as the Université de Montréal and the University of Bonn working collaboratively, the prospects for cleaner, energy-efficient chemical processes look promising. This work not only opens doors for innovations in green chemistry but also empowers society to move towards environmentally sustainable industrial practices. As we strive for a more circular economy, such novel approaches may well rewrite the future of chemical manufacturing.

Leave a Reply