Pancreatic cancer has long been known as one of the most challenging forms of cancer to detect early on, leading to poor prognoses for many patients. The current markers used for screenings are often unreliable and fail to catch the disease in its initial stages. However, a recent study published in the journal Angewandte Chemie has introduced a promising new method for diagnosing pancreatic cancer with greater precision and accuracy.
The Role of Tumor-Associated Autoantibodies
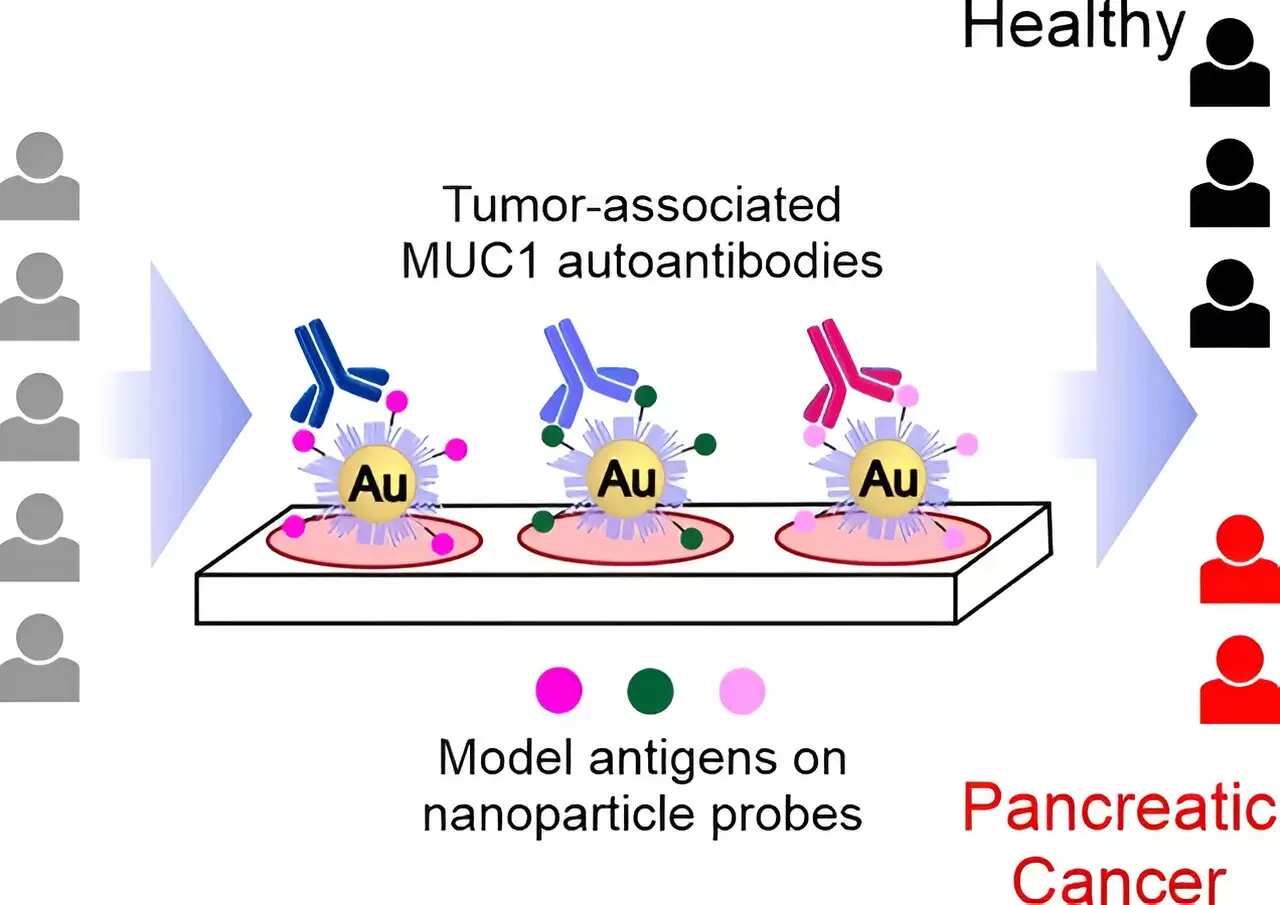
Tumors release specific proteins known as tumor-associated antigens, which stimulate the immune system to produce antibodies targeted against the tumors. These antibodies, called tumor-associated autoantibodies, can be detected in blood samples at the early stages of the disease, making them valuable indicators for early detection. A team of researchers led by Roberto Fiammengo and Giovanni Malerba at the University of Verona, along with other international collaborators, developed a diagnostic approach based on the detection of these tumor-associated autoantibodies.
Focus on TA-MUC1
The researchers chose to target autoantibodies directed against the tumor-associated form of mucin-1 (TA-MUC1), a glycosylated protein found in elevated levels in many types of tumors, including pancreatic cancer. By structurally analyzing known antibodies against TA-MUC1 and designing synthetic glycopeptides that mimic different segments of TA-MUC1, the team created probes suitable for detecting the presence of specific autoantibodies indicative of pancreatic cancer.
The team validated their diagnostic assay using samples from patients with pancreatic cancer and a healthy control group. The nanoparticle probes designed by the researchers were able to differentiate effectively between samples from diseased individuals and healthy controls, demonstrating their ability to detect tumor-associated autoantibodies with high accuracy. Importantly, these specific autoantibodies showed better positive/false positive ratios compared to current clinical biomarkers for pancreatic cancer.
The researchers found that probes with smaller glycopeptide antigens, corresponding to single epitopes of TA-MUC1, performed better than larger probes mimicking multiple epitopes. This not only improved the accuracy of the diagnostic assay but also made the production of synthetic probes easier. A short glycopeptide with an unnatural modification to its sugar component was particularly effective in detecting discriminating autoantibodies, highlighting the potential of this new method for selecting autoantibody subgroups with higher tumor specificity.
The development of a structure-based approach for detecting tumor-associated autoantibodies in pancreatic cancer represents a significant advancement in early diagnosis. By leveraging the immune response to specific antigens, researchers have created a more reliable method for identifying the disease in its early stages. This innovative approach has the potential to revolutionize pancreatic cancer diagnosis and improve outcomes for patients.
Leave a Reply