Noble gases have long been celebrated for their lack of reactivity, often labeled as inert elements that resist forming compounds. This perception changed dramatically over sixty years ago when Neil Bartlett made a groundbreaking discovery by successfully bonding xenon to create the compound XePtF6. This orange-yellow solid marked a pivotal moment in chemistry, redefining our understanding of noble gases. Since Bartlett’s initial experiment, numerous noble gas compounds have been synthesized, though many remain poorly understood due to difficulties in crystal growth and the inherent instability of these compounds.
The characterization of noble gas compounds presents unique challenges. X-ray diffraction, a common technique used to analyze crystal structures, often struggles with the delicate nature of noble gas-containing crystals, which are highly susceptible to moisture and other environmental factors. These compounds require careful handling and specialized techniques for crystallization to obtain samples large enough for reliable analysis. Consequently, many of the fascinating structural features of these compounds have remained undiscovered or poorly characterized.
Emerging Techniques in Crystallography
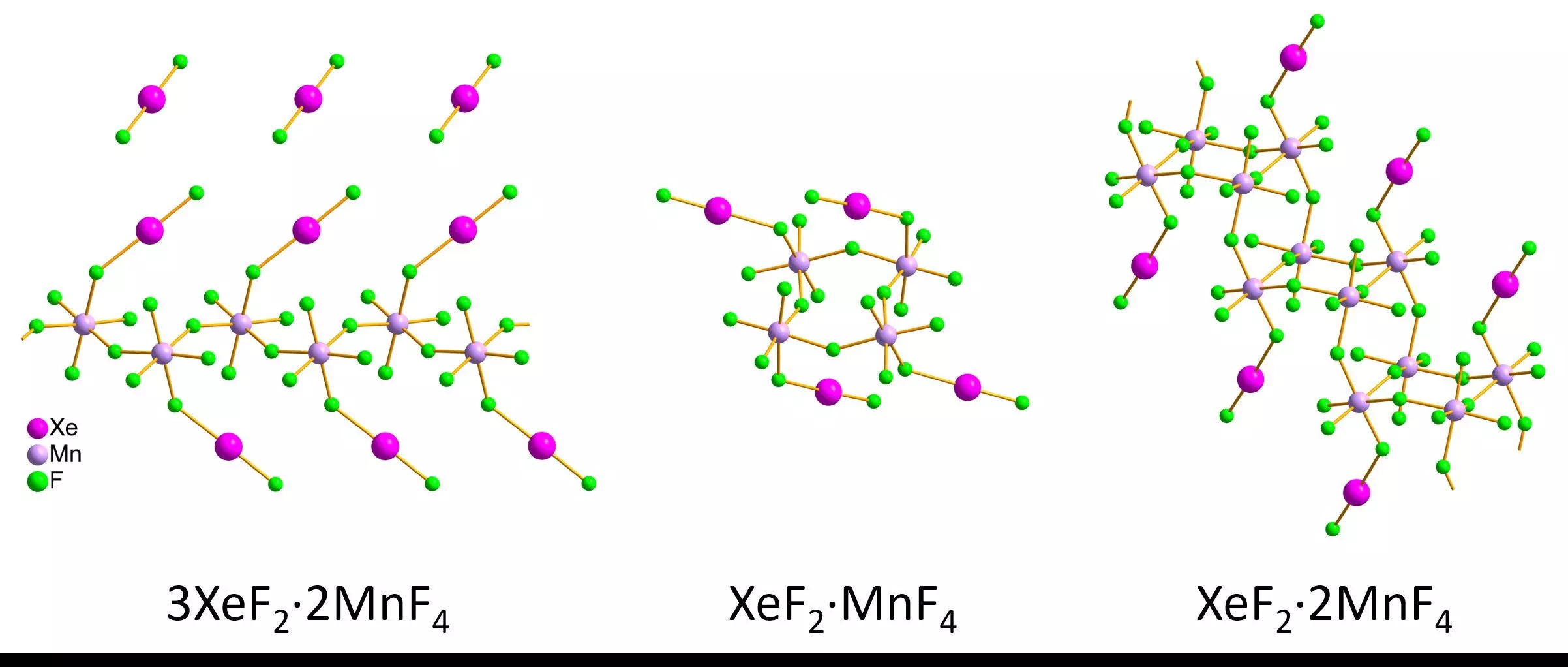
Recent advances in electron diffraction have opened new avenues for studying noble gases. A cutting-edge method known as 3D electron diffraction has emerged, allowing researchers to explore the structures of nanoscale crystals, which are often more stable in air than their larger counterparts. This innovative technique offers the promise of overcoming previous limitations associated with air-sensitive compounds. In a notable study led by Lukáš Palatinus and Matic Lozinšek, researchers turned to 3D electron diffraction to examine various xenon difluoride-manganese tetrafluoride compounds. By synthesizing red and pink crystals and using a liquid nitrogen cooling technique to stabilize their samples, they successfully characterized the intricate bond lengths and angles within these compounds.
Results that Resonate
The researchers recorded their findings, observing distinct structural configurations among different compounds. The 3D electron diffraction revealed infinite zigzag chains for the compound 3XeF2·2MnF4, cyclic arrangements for XeF2·MnF4, and unique staircase-like double chains for XeF2·2MnF4. Astonishingly, the results obtained from 3D electron diffraction closely aligned with earlier measurements taken from larger, micrometer-sized crystals analyzed via single-crystal X-ray diffraction. This consistency bolsters confidence in the reliability of the 3D electron diffraction technique, signaling a breakthrough in the study of noble gas compounds.
The successful application of 3D electron diffraction to study xenon-containing compounds holds profound implications for future research. With this method, scientists now have a valuable tool to investigate the structural complexities of historically challenging noble gas compounds, including the elusive XePtF6. As researchers continue to refine these techniques, the potential to uncover new and previously uncharacterized compounds may expand our understanding of not just noble gases, but a wide array of air-sensitive materials as well. This line of inquiry reaffirms the importance of innovative methodologies in driving forward the frontiers of modern chemistry.

Leave a Reply