Seawater electrolysis presents a groundbreaking opportunity to decarbonize the energy sector, targeting sustainable hydrogen production through the utilization of abundant seawater. The method promises a pathway to generate clean fuels but is currently impeded by critical challenges. Factors such as anode corrosion caused by chloride ions, the initiation of unwanted oxidation reactions, and the prohibitive costs of catalysts have significantly slowed progress in this field. Addressing these obstacles is paramount for the realization of efficient seawater electrolysis.
In recent times, self-supported nickel-iron (NiFe) materials have emerged as promising bifunctional catalysts for both hydrogen evolution (HER) and oxygen evolution reactions (OER). Their widespread appeal stems from their affordability and high intrinsic activity. However, these catalysts face significant hurdles, primarily in corrosive environments typical of seawater. The innovative application of wood-based carbon (WC) materials has garnered attention due to their advantageous hierarchical porous structure and excellent electrical conductivity. This unique combination makes WC an ideal substrate for embedding active catalysts, particularly in harsh electrochemical environments.
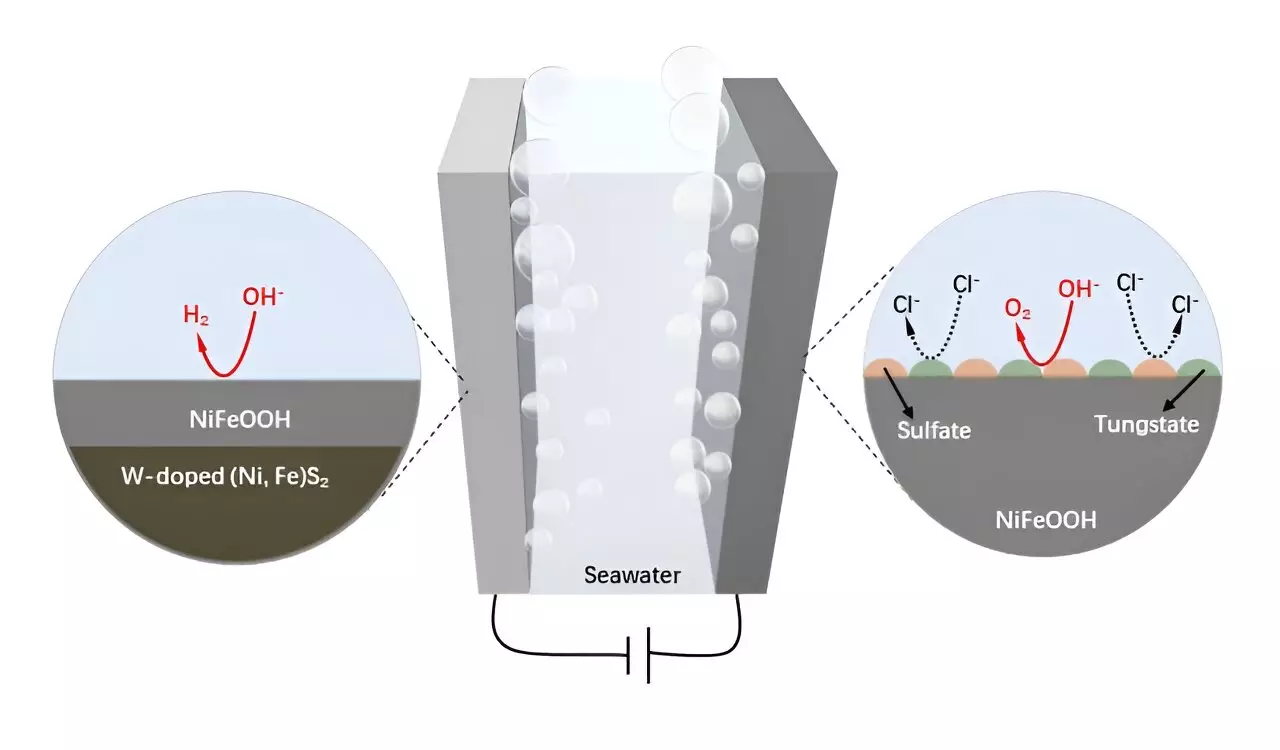
A team of researchers led by Prof. Hong Chen from the Southern University of Science and Technology, along with colleagues from Australia, has taken a significant step forward in enhancing the stability and efficiency of NiFe-based electrodes. As detailed in their publication in the *Science Bulletin*, the introduction of tungsten into the NiFe catalysts resulted in substantial improvements in corrosion resistance and electrode longevity. The newly developed W-doped NiFe sulfide (W-NiFeS) supported on wood-based carbon (WC) stands out due to its innovative design and preparation method, which includes a process of impregnation and sulfidation.
The W-NiFeS/WC electrode showcases an impressive three-dimensional porous configuration characterized by oriented microchannels and densely anchored W-NiFeS nanoparticles. This complex structure not only enhances the electrode’s electrical conductivity but also supports superior efficiency during seawater electrolysis. The material’s innovative design promotes rich redox-active centers, contributing to remarkable electrocatalytic activities and prolonged stability when subjected to alkaline seawater conditions.
The implications of this research extend beyond technical advancements in electrochemistry. The team’s work also champions a circular economy mindset by utilizing sustainable materials—in this case, wood waste—to create advanced catalytic structures. Such an approach minimizes waste production while simultaneously fostering a robust framework for green hydrogen production from seawater. This undertaking not only reflects a significant leap in sustainable technologies but also emphasizes the adaptability and reusability of resources in the quest for renewable energy solutions.
The research spearheaded by the team highlights the potential of innovative materials and structures to redefine seawater electrolysis efficiency. This work sets the stage for further developments in the field, steering us towards sustainable and economically viable hydrogen production techniques that can significantly reduce reliance on fossil fuels.

Leave a Reply