As the world shifts toward sustainable energy solutions, hydrogen emerges as a potential linchpin in decarbonizing various industries. Its advantages lie in being a clean fuel that can power vehicles, generate electricity, and serve as a key component in numerous chemical processes. However, one of the most pressing issues surrounding hydrogen usage is its volatility and the challenges associated with safe storage. Researchers from the Leibniz Institute for Catalysis (LIKAT) in Rostock, in collaboration with H2APEX, have taken significant steps to address this concern. Their recent findings, published in *Nature Communications*, reveal an innovative approach to chemically store hydrogen using simple and safe materials.
The researchers developed a groundbreaking homogeneous catalyst system that effectively binds hydrogen (H2) to potassium bicarbonate, a stable and safe compound often recognized as baking soda. This method enables the chemical storage of hydrogen in a manageable form, mitigating the risks associated with hydrogen’s inherent volatility. At the crux of this chemistry is a ruthenium catalyst that facilitates the reaction, allowing hydrogen to interact with bicarbonate and transform into formate, a benign salt derived from formic acid.
Dr. Rui Sang and Ph.D. candidate Carolin Stein, the study’s primary authors, emphasize the reversible nature of this storage method. The implications are profound: “We can release the hydrogen stored in the formate again at any time — with the same catalyst, in the same system.” This reversible reaction mirrors the dynamics of a battery, making it a promising candidate for energy storage systems.
The hydrogen-binding system operates efficiently at approximately 60°C, optimizing chemical reactions in a single solution where all reactive components are present. This setup simplifies the storage process, enabling straightforward temperature control to dictate the hydrogen’s binding and releasing actions. The system’s operational versatility is highlighted by Dr. Peter Sponholz of H2APEX, who explains how the pressure at which hydrogen is added can direct the process in either direction. This unique adaptability positions formate as a flexible energy storage medium.
Through extensive experimentation, the researchers discovered the pivotal role that the storage medium’s properties play in maximizing hydrogen storage capabilities. By utilizing potassium and carefully choosing potassium bicarbonate, they engineered an optimal salt for the process. This decision underscores the importance of selecting suitable cations and anions in optimizing hydrogen storage efficiency.
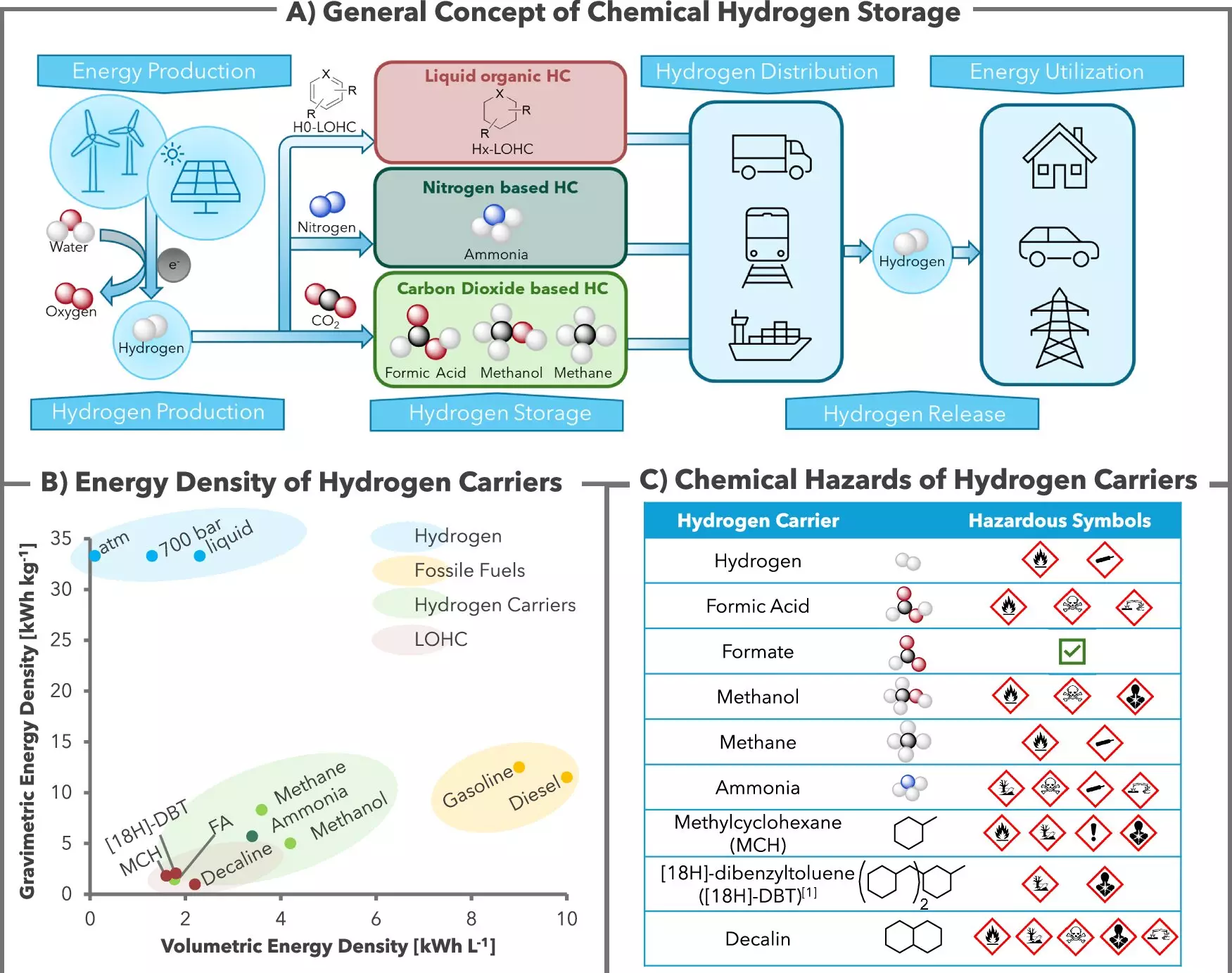
The emerging hydrogen economy has often pointed towards alternatives such as methanol, ammonia, and methane for hydrogen storage. However, formic acid salts offer distinct advantages, especially regarding toxicity and energy consumption. As Henrik Junge articulated, formate could be stored similarly to conventional liquids such as milk or diesel, facilitating transportation in standard containers. This practicality is a crucial aspect of integrating hydrogen into energy infrastructures, particularly in rural regions where energy sourcing can be challenging.
Furthermore, the approach ties into renewable energy generation, particularly from intermittent sources like wind and solar power. When surplus electricity is produced during periods of high supply, it’s feasible to use that energy to create green hydrogen via electrolysis and store it as formate. This interplay reflects a step towards sustainable energy practices that align with environmental goals.
A remarkable facet of this hydrogen storage technology is its CO2-neutral process. Conventional methods may unintentionally release carbon dioxide during hydrogen recovery, exacerbating environmental concerns. In contrast, this new system keeps CO2 permanently stored along with hydrogen, thereby enabling access to pure hydrogen suitable for fuel cell applications without purification.
In practical tests, the researchers successfully executed 40 consecutive cycles of hydrogen storage and release over six months. Remarkably, they produced 50 liters of hydrogen with an impressive purity level of 99.5% using minimal amounts of ruthenium catalyst. This efficiency showcases the potential of their findings and presents a pathway to commercial applications.
The partnership between LIKAT and H2APEX is poised to revolutionize how we think about hydrogen. With plans to develop a larger demonstrator plant expected to be commercialized by the end of 2025, the quest for a stable, efficient, and safe hydrogen storage solution continues to unfold. As the story of hydrogen storage evolves, the symbol “H” not only represents hydrogen atoms but also a hopeful future for sustainable energy transitions worldwide. With continued dedication to research and innovation, the dream of a hydrogen-powered economy could soon become a reality.
Leave a Reply