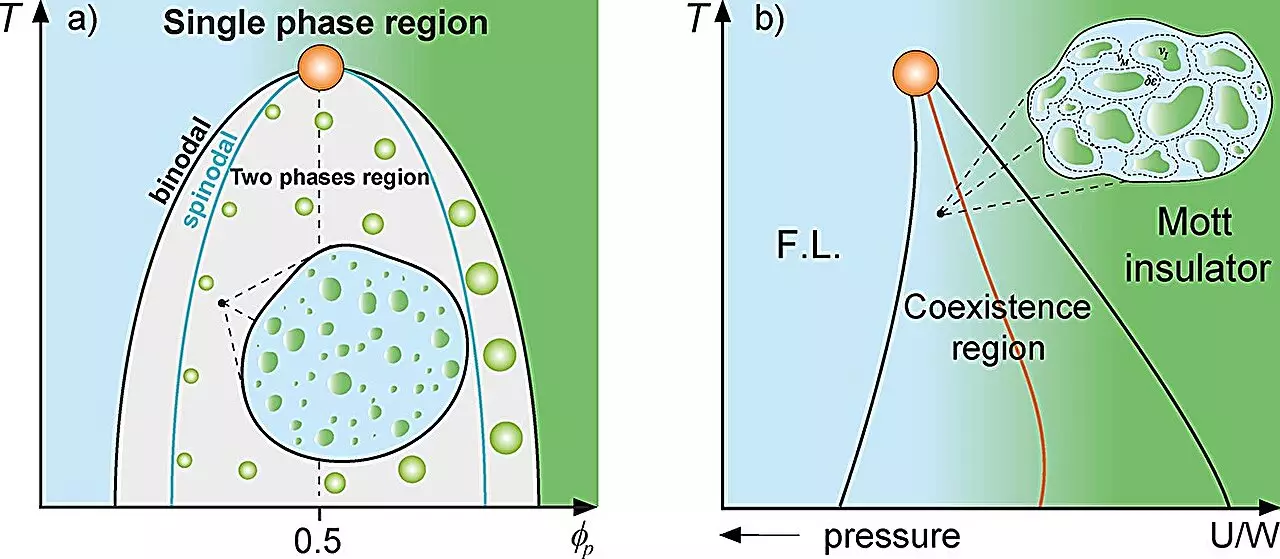
In the realm of physics, the classical mixture theory sheds light on systems composed of multiple substances. This approach allows scientists to consider the fractional presence of each substance and the various interactions at play. Notably, this theoretical framework finds application in understanding diverse phenomena, from the behavior of supercooled water displaying multiple density phases to the intriguing characteristics of metals transitioning between conducting and insulating states. Such investigations have led researchers to explore analogous situations within biological systems, particularly the compartmentalization of proteins within cellular environments.
Recent research conducted by a team at São Paulo State University (UNESP) has delved into the dynamics of protein compartmentalization by employing principles from condensed matter physics. The findings, published in the journal *Heliyon*, propose a unique perspective: the existence of a Griffiths-like phase within cells. This concept draws parallels between the behavior of protein droplets and the magnetic Griffiths phase, where distinct regions emerge within a matrix. Such rare regions significantly impact the kinetics and dynamics of the system—an essential feature mirrored in cellular activity.
The study, led by Professor Mariano de Souza and Ph.D. candidate Lucas Squillante, highlights how the presence of protein droplets can significantly hinder cellular dynamics as they approach a threshold for liquid-liquid phase separation. This phenomenon can be likened to the dynamics observed in magnetic systems where regions of differing magnetization create a complex interplay that affects overall behavior.
To provide a deeper understanding of the observed phenomena, the research team employed various thermodynamic models. Their analysis included the Grüneisen parameter and the Flory-Huggins model, which are instrumental in depicting the phase interaction between proteins and their solvent. They demonstrated that the reduced dynamics near the binodal line—where phase separation occurs—affects the protein’s concentration and subsequently alters their mobility within the cytoplasm, suggesting the emergence of a Griffiths-like cellular phase.
Through this framework, the study not only advances knowledge about protein dynamics but also hints at evolutionary implications. The researchers propose that such coacervates—droplets formed from organic molecules—play a pivotal role in primordial life development, supporting theories proposed by biologist Aleksandr Oparin regarding the chemical origin of life.
A fascinating aspect of the study connects the concept of homochirality—where one chirality predominates in biological molecules—to the dynamics of protein interaction and compartmentalization. This characteristic may have been crucial for the survival and adaptation of early life forms, emphasizing the importance of specific molecular structures in biological evolution. The researchers argue that an increase in protein diffusion time corresponds to reduced stochastic fluctuations, optimally facilitating gene expression.
This perspective opens new avenues for understanding how such physical properties influence molecular biology. It suggests that cellular dynamics, which can become critical in pathological conditions, are tightly woven into the fabric of cellular evolution.
The relevance of liquid-liquid phase separation extends beyond fundamental biology; it has significant implications for understanding and treating diseases. As highlighted by co-author Professor Marcos Minicucci, the role of compartmentalization is coming under increasing scrutiny in the context of diseases, including cancer, neurodegenerative disorders, and various conditions arising from protein misfolding.
The concept of a Griffiths-like cellular phase could illuminate how aberrations in protein dynamics contribute to disease processes, possibly affecting cell mutations or impairing functions that lead to, for instance, tumor progression. Insights from this research could spur innovative approaches to therapeutic intervention, utilizing the principles of phase separation to inform the development of new treatment strategies.
The findings from the UNESP team underscore the value of interdisciplinary research, merging insights from physics, biology, and medicine to address complex biological phenomena. As our understanding of protein dynamics and their implications deepens, it becomes evident that the interplay between physical principles and biological processes is key to unraveling the mysteries of life and disease.
The research into Griffiths-like phases not only enriches our comprehension of cellular mechanics but also paves the way for future studies aimed at manipulating these processes for therapeutic purposes. By bridging gaps between disciplines, scientists can devise comprehensive strategies to tackle diseases grounded in the fundamental laws of physics and biology alike.
Leave a Reply