In a significant stride toward developing more effective treatments for aggressive cancers, researchers from Oak Ridge National Laboratory (ORNL) have achieved remarkable insights into the workings of the enzyme serine hydroxymethyltransferase (SHMT). Utilizing neutron experiments, the team uncovered detailed atomic interactions within this crucial metabolic enzyme, instrumental in cell division and cancer proliferation. This innovative research promises to pave the way for targeted drug design, particularly inhibitors that could cripple cancer cells by blocking specific metabolic pathways.
Cancer’s ability to manipulate and hijack metabolic processes enables it to thrive and proliferate uncontrollably. The pathway that SHMT participates in is known as one-carbon metabolism—essential for synthesizing nucleic acids and other biological molecules. This pathway is integral to producing DNA and RNA, which are vital for cell division. The research highlights how cancer cells remold this metabolic pathway to support their relentless growth. In particular, if scientists can design an effective inhibitor that targets SHMT, they may be able to halt cancer’s advances at an early stage in the one-carbon pathway, potentially leading to more effective treatment outcomes.
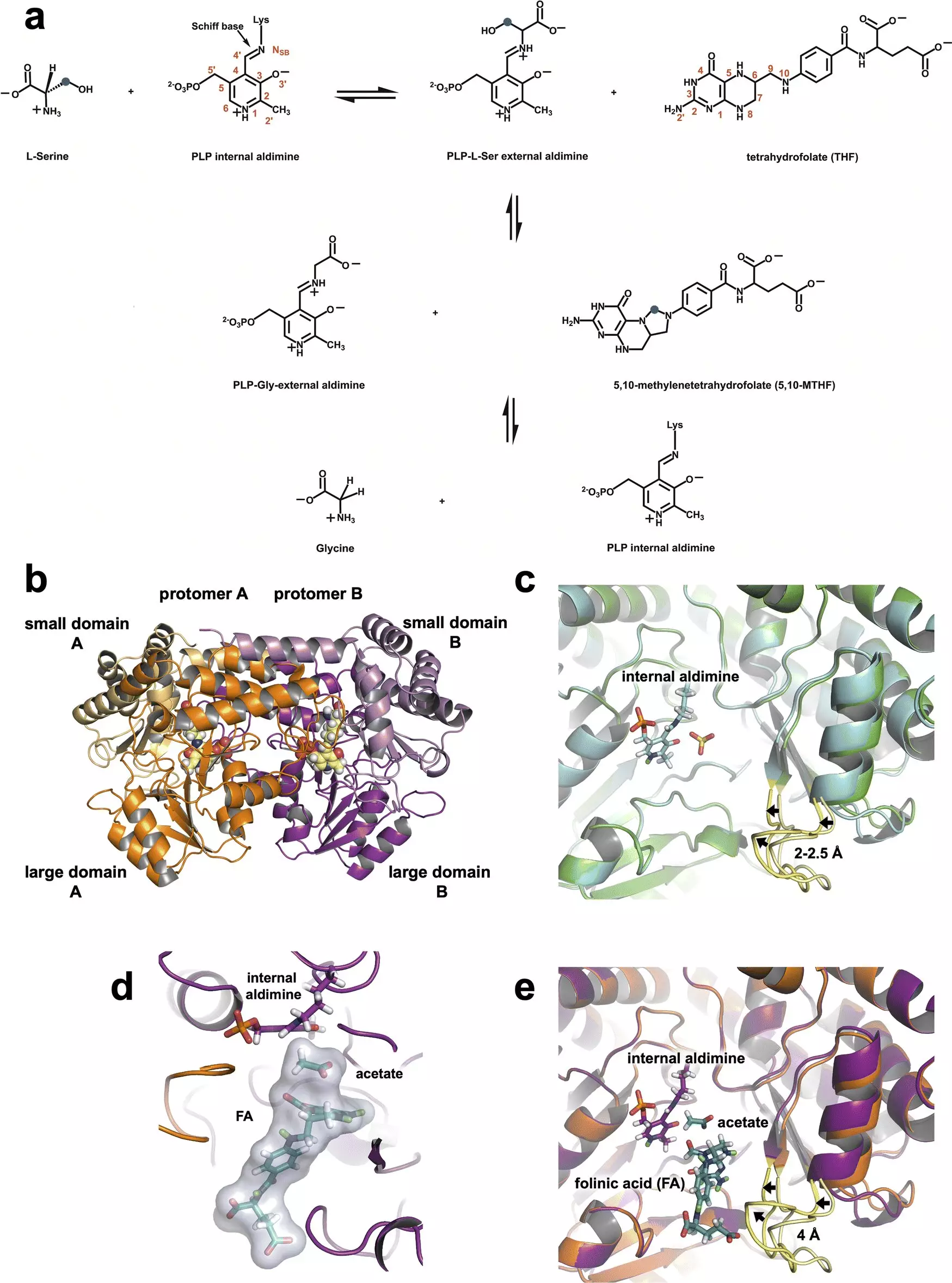
The current study, published in the journal Chemical Science, builds upon decades of research that have aspired to unravel SHMT’s exact catalytic mechanism. By employing neutron diffraction techniques at both the Spallation Neutron Source and the High Flux Isotope Reactor, the researchers captured atomic-level details that were previously elusive. The neutron experiments illuminated not just the enzyme’s structure but revealed the pivotal role of a specific amino acid residue—glutamate. This residue appears to act as a crucial regulatory element within the enzyme’s active site, managing proton transfers necessary for the enzyme’s function.
Lead author Victoria Drago notes the importance of neutron data, stating, “Neutrons allow us to see hydrogen atoms, and hydrogen drives chemistry.” This capability is particularly valuable in biochemical studies as hydrogen plays a fundamental role in enzymatic reactions. The researchers demonstrated that the glutamate residue maintains its proton, allowing it to function both as an acid and a base, effectively facilitating the enzyme’s catalytic activity.
The collaboration at ORNL employed advanced techniques, such as combining neutron and X-ray crystallography, to discern the structural characteristics of SHMT and its interactions with related compounds like tetrahydrofolate—an essential cofactor in the reaction. This multi-faceted approach reflects the complexity inherent in biological systems and the difficulty of targeting enzymes that are crucial both for normal cellular function and cancer proliferation.
Through detailed observation, the research team established the mechanism by which SHMT converts serine to glycine. The intricate interactions of the amino acids involved in this process were mapped, shedding light on how cancer cells might exploit these reactions for their benefit. With this detailed understanding, the team aims to create a specialized inhibitor that would disrupt the catalytic function of SHMT, thereby blocking the pathway essential for cancer cell growth.
The implications of this research extend beyond mere academic interest. As current cancer therapies often indiscriminately affect both malignant and healthy cells, leading to significant side effects, understanding the specific actions of enzymes like SHMT can revolutionize treatment strategies. By designing drugs that specifically target SHMT’s functions, researchers hope to minimize collateral damage to healthy tissues, improving patient outcomes.
Moreover, the advancements in neutron science, backed by ORNL’s recent Proton Power Upgrade, signify a leap forward in our capacity to gather data quickly and efficiently. These improvements enhance the feasibility of employing neutrons in drug discovery and development, making this method increasingly appealing for future research in structure-based drug design.
The meticulous investigation of SHMT through neutron experiments marks a significant leap forward in our understanding of cancer metabolism and enzyme function. This research is poised to inform the design of innovative cancer therapies that target metabolic pathways with unprecedented precision. The promise of neutron diffraction in revealing atomic-level details solidifies its place as a crucial tool in the ongoing battle against cancer, potentially leading us closer to tailored treatments that offer hope for better survival rates and fewer side effects for patients. As the landscape of cancer research evolves with advancements in technology, the opportunity for transforming patient care is brighter than ever before.

Leave a Reply