In the ever-evolving field of synthetic chemistry, notable breakthroughs often pave the way for groundbreaking applications in drug discovery and development. A recent study out of Tokyo Institute of Technology, involving a team led by Assistant Professor Yuki Nagashima, exemplifies such innovation. This research highlights a transformative method for synthesizing complex organic molecules known as 2D/3D fused frameworks, utilizing inexpensive quinoline derivatives as starting materials. Their pioneering technique combines simplicity and cost-effectiveness, potentially altering the landscape of pharmaceutical synthesis by enabling highly customizable drug candidates.
Harnessing the Power of Quinolines
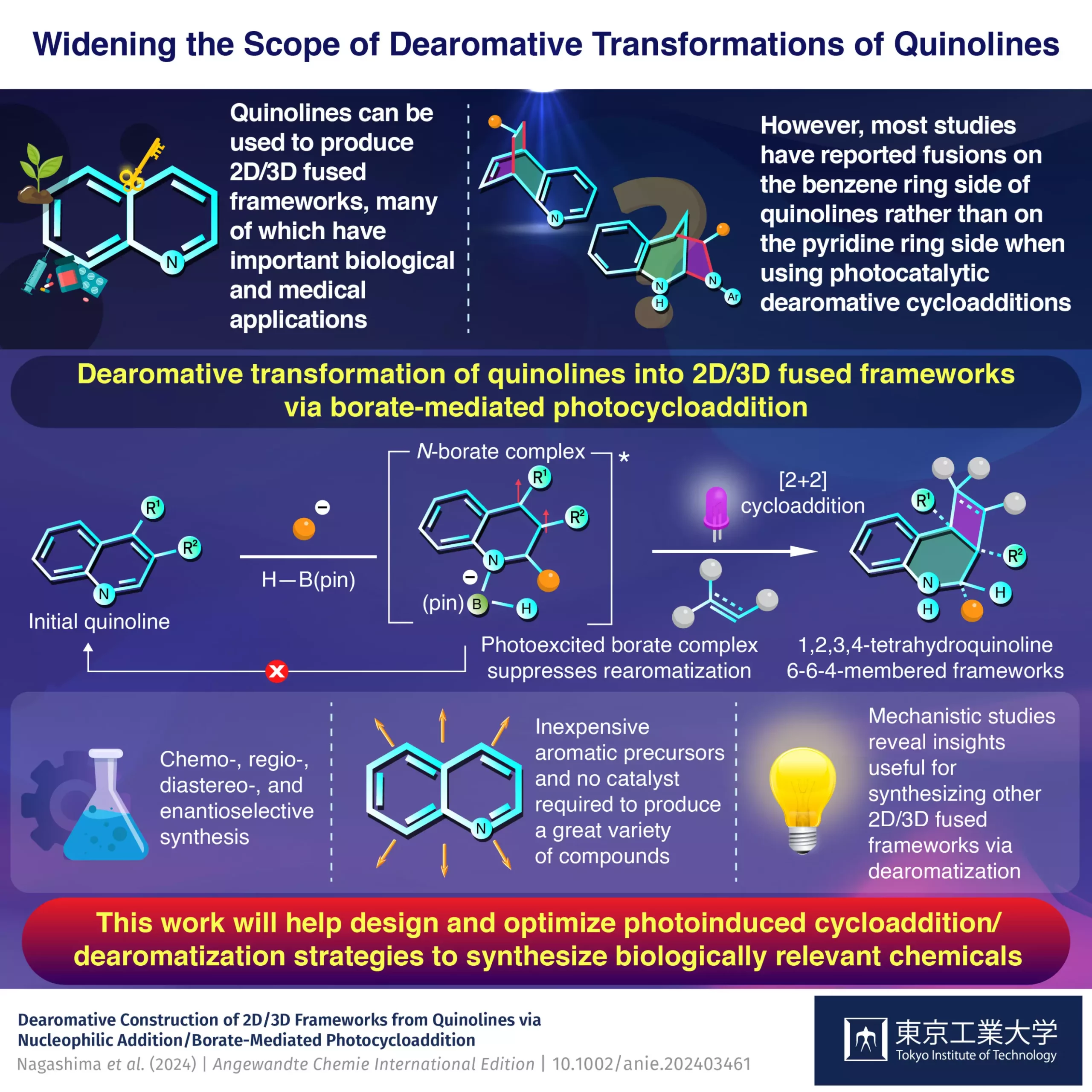
Quinolines have emerged as a significant focus for chemists interested in producing 2D/3D frameworks due to their distinctive electronic properties. The structure of quinolines consists of a benzene ring that is rich in electrons, fused with a pyridine ring that is electron-poor. This unique setup allows for independent modification of the two rings, offering a rich vein of potential compound diversity. By manipulating reaction conditions, chemists can create various chemical entities with specific functionalities, which is particularly valuable in tailor-making drug candidates.
Despite the promising nature of quinolines, most conventional methods have primarily concentrated on modifying the electron-rich benzene side of the molecule. Pioneering studies seldom ventured into the realm of the pyridine side, thus leaving a treasure trove of possibilities largely unexplored. The Tokyo Tech team sought to change that narrative by devising a strategy that effectively targets the pyridine ring, thus expanding the toolkit available to synthetic chemists.
Novel Mechanisms: The Role of Photocycloaddition
Central to this innovative methodology is the use of dearomative photocycloadditions, a process that destabilizes the aromatic nature of the quinoline’s rings using light alongside a critical boron-containing intermediate known as pinacolborane. This methodology enables the coupling of reactants onto the pyridine side, marking a significant departure from prior techniques that had little success in this area.
Through their research, the Tokyo Tech team found that the pinacolborane compound not only effectively facilitates these reactions but also does so with heightened efficiency, resulting in high yields across a variety of quinoline derivatives. The researchers conducted extensive experiments and theoretical analyses to better understand the mechanisms at play. They discovered that the photochemical reaction occurs in two primary steps, first involving an organolithium compound followed by the formation of a borate complex. This borate complex serves to accelerate the cycloaddition while simultaneously inhibiting the unintended return to aromatic forms, a common issue in earlier methodologies.
Breaking New Ground in Organic Synthesis
The ramifications of this breakthrough are substantial. Traditional synthetic approaches often involve cumbersome steps and high costs—challenges that the new strategy appears to mitigate effectively. Not only does it require fewer reaction steps, but it also eliminates the need for catalysts, allowing researchers to save both time and financial resources. Importantly, the ability to employ multi-substituted starting materials further enhances the accessibility of a wide range of target compounds, providing chemists with unprecedented versatility.
As Nagashima reflects on their findings, he emphasizes the game-changing nature of this work: “Our detailed mechanistic studies revealed that the photoexcited borate complex both accelerates the cycloaddition and suppresses the rearomatization that usually occurs in conventional photocycloaddition reactions.” The utility of this strategy for the further functionalization of diverse aromatic compounds signals not simply a refinement of existing techniques, but a reimagining of what is possible within organic synthesis.
The Future of Drug Development
The exploration of 2D/3D fused frameworks represents more than just an academic endeavor; it holds the promise of driving forward the next generation of pharmaceuticals. As researchers begin to tap into the potential of quinolines, we may find ourselves on the cusp of a new era in the development of organic compounds tailored for medicinal use. The implications of such a capability could be revolutionary, especially in the realm of personalized medicine where the precision of drug design is of paramount importance.
The innovative approach detailed by Nagashima and his colleagues may not only unlock the underutilized potential of quinolines but also empower chemists to synthesize a broader array of compounds than previously thought possible. The future of synthetic chemistry is ripe for exploration, with the promise of new methodologies leading to groundbreaking advancements in drug discovery.

Leave a Reply