Gallium, a relatively obscure element discovered in 1875 by the French chemist Paul-Émile Lecoq de Boisbaudran, has long captivated the scientific community due to its unusual properties. Characterized by a melting point low enough that a spoon made of gallium will dissolve in a cup of tea, this metal occupies an essential niche in the world of semiconductors and advanced material science. Recently, a groundbreaking study conducted by scientists at the University of Auckland revealed intriguing insights into gallium’s atomic structure and behavior, challenging preconceived notions that have persisted for decades.
The breakthrough hinges on the way gallium behaves at the atomic level, which distinguishes it sharply from traditional metals. Gallium exists primarily as ‘dimers’—pairs of atoms that exhibit covalent bonding, a rarity among metals known for predominantly ionic or metallic bonds. Understanding these properties is not merely an academic exercise; it has real implications for nanotechnology and materials science as researchers look to harness the unique attributes of gallium in innovative applications.
A Paradigm Shift in the Understanding of Metal Properties
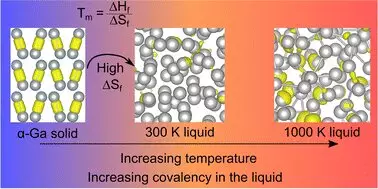
The paper titled “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium,” published in *Materials Horizons*, challenges the foundational assumptions that have guided scientific thought about this element for thirty years. Professor Nicola Gaston of the University of Auckland summarized the significance of this work, eloquently stating that the established view of gallium’s liquid structure, which depended on the existence of covalent bonds, has proven to be fundamentally flawed.
The key revelation is that these covalent bonds disappear at the melting point of gallium but suddenly reappear at higher temperatures. This peculiar behavior opens the door to a deeper understanding of “entropy”—essentially, a measure of disorder within a system. The study proposes that when the bonds disappear upon melting, the increased entropy allows gallium atoms greater freedom of movement, fundamentally altering its physical characteristics.
This revolutionary perspective prompts researchers to reevaluate how they interpret the melting points and structural dynamics of not only gallium but potentially other metals.
Implications for Nanotechnology and Beyond
The implications of this new understanding are profound and multifaceted. Gallium’s unique properties are instrumental in developing self-assembling structures and liquid metal catalysts—elements crucial for advancements in nanotechnology where precision at the atomic level is paramount. The historic collaboration among Dr. Steph Lambie, Professor Gaston, and Dr. Krista Steenbergen showcases a meticulous effort to reassess existing literature critically and incorporate temperature data, leading to a comprehensive understanding of gallium’s characteristics.
The metal’s usage spans a variety of high-tech industries, including telecommunications, aerospace, and even renewable energy sectors through solar panels. Interestingly, gallium is also being investigated for its potential to aid in astrobiology. Scientists exploring the Martian surface hope that gallium can serve as a chemical ‘fingerprint’ that preserves traces of past microbial life, underscoring the element’s versatility and relevance in contemporary research.
A Legacy that Continues to Shape Our Future
As we honor gallium’s role in the periodic table and its applications, it’s equally important to note its predictive history. Dmitri Mendeleev left gaps in his first periodic table for elements yet to be discovered, demonstrating an uncanny foresight and understanding of atomic interactions. Gallium’s eventual discovery after Mendeleev’s conceptualization is a testament to the complexities of chemical science and the ongoing quest to piece together the puzzle of the universe at an atomic level.
The dedication of researchers to uncover new facets of materials like gallium is what drives the realm of scientific discovery. The journey of gallium, from its curious melting point that perplexed chemists to its potential as a key player in the electronics revolution, embodies the beauty of discovery in science—where each finding upends traditional wisdom and inspires new lines of inquiry. Through such work, we continue to unlock the mysteries of matter, potentially leading us toward even greater innovations that could reshape our understanding of the world.

Leave a Reply