In nature, ice rarely exists in isolation; it is nearly always surrounded by liquid water. This perpetual interaction gives rise to complex physical phenomena that profoundly affect many aspects of our environment—from climate systems to everyday experiences like skating on ice or enjoying ice cream. Despite its ubiquity, the microscopic relationship between ice and liquid water has remained largely enigmatic until recently. A pivotal study spearheaded by researchers at Kobe University and the Institute for Molecular Science has ushered in a new era of exploration by directly observing the ice-liquid boundary for the first time.
A Novel Approach to Observation
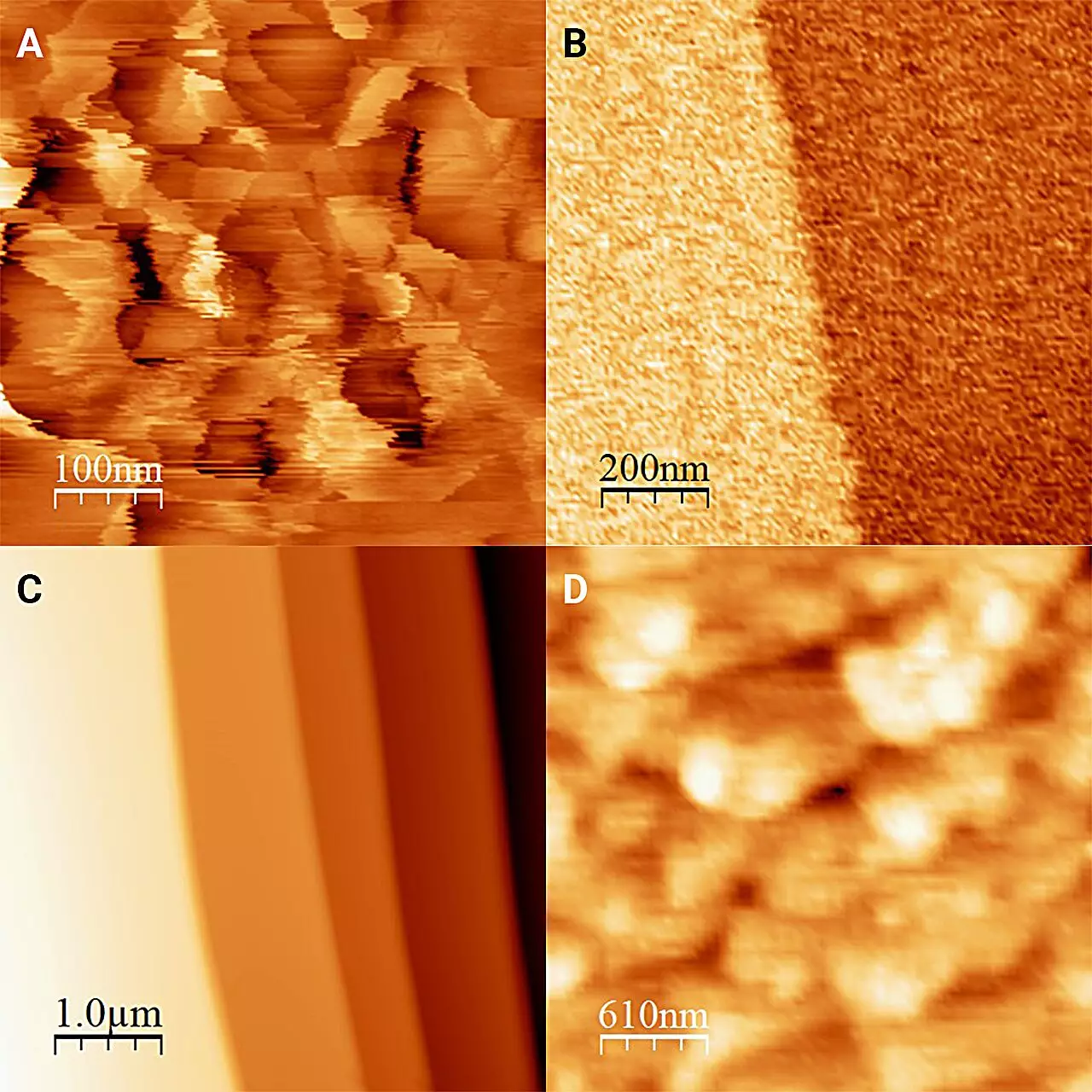
Traditional methods of studying the ice-water interface have been thwarted by the rapid phase changes between ice and water, making it nearly impossible to capture a stationary view. This research overcame this limitation through an innovative approach involving antifreeze and a refrigerated microscope. Lead researcher Onishi Hiroshi pointed out that immersing ice in an antifreeze medium below 0°C allows for a stable interface that doesn’t change or melt, facilitating precise observations. This idea displays a critical understanding of material states and the necessity of creative thinking in overcoming experimental challenges.
The team faced significant hurdles, particularly in ensuring the stability of their atomic force microscope under sub-zero conditions. They had to engineer solutions that allowed for effective cooling of the entire microscope system while maintaining its accuracy. This showcases not only the ingenuity required in scientific endeavors but also the lengths to which researchers must go to push the boundaries of knowledge.
Key Findings and Implications
The findings published in The Journal of Chemical Physics shed light on the structural characteristics of the ice surface when it interacts with liquid. When the researchers observed ice in the antifreeze, they noted that the ice surface was perfectly flat, interspersed with minute steps of just one molecular layer high. In contrast, ice without the influence of surrounding liquid displayed formations known as “frost pillars” standing at 20 nanometers. This fundamental difference in structure highlights the transformative nature of liquid on solid surfaces—a crucial insight that could reshape our understanding of environmental systems and various industrial processes.
The research also explored the behavior of ice with various alcohols as antifreeze, revealing that even slight differences in liquid properties could produce markedly different ice surfaces. This nuance illustrates the critical need for direct measurements at the molecular level when studying interfaces. Each liquid creates a unique interaction with the ice, emphasizing that not all liquids are equal in their effects—an assertion that holds significant implications for both scientific inquiry and practical applications.
Furthermore, the researchers discovered that the “hardness” of ice is more considerable than previously thought, which could lead to new understandings of how ice behaves in environments like polar regions or engineered ice structures. This advancement paves the way for applications not only in understanding climate dynamics but also in improving technologies that rely on ice, such as winter sport equipment or ice-defrosting methods in various industries.
Future Directions in Ice Research
With their groundbreaking results, Onishi and his team have opened avenues for future research that extend beyond simple observations. They aspire to enhance the resolution of their measurements down to the single water molecule level, employing methodologies that surpass atomic force microscopy. This ambition is as exciting as it is challenging, signaling a step towards an even deeper understanding of molecular interactions at play in nature.
An exploration of the ice-liquid interface is not solely an academic exercise; the ramifications stand to affect numerous fields, from climatology to materials science. As we continue to study ice through the lens of innovative techniques, we may gain insights not only about our planet’s climate and environmental challenges but also about material resilience and engineering solutions for extreme conditions.
Ultimately, this research reminds us that profound advancements often stem from an interplay of creativity, technology, and relentless inquiry. The world of ice—once perceived as simple and unyielding—reveals itself as a complex and dynamic element of our reality, one that we have only begun to unravel.

Leave a Reply