In a groundbreaking study led by Prof. Chen Changlun from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, significant advancements have been made in the realm of hydrogen production through water electrolysis. The research team has successfully developed cobalt-doped nickel hydroxide bipolar electrodes and innovative non-noble metal catalysts, both of which play a pivotal role in enhancing the efficiency and stability of a sophisticated two-step water electrolysis process. This novel approach showcases potential as a beacon of hope for sustainable energy solutions amid growing concerns about fossil fuel dependency and environmental sustainability.
Challenges in Traditional Electrolysis
Traditional alkaline electrolyzers are fraught with limitations, primarily stemming from their mismatch with the highly variable nature of renewable energy sources. Additionally, issues of hydrogen and oxygen mixing under high-pressure conditions hinder their practicality. Pioneering researchers have identified two-step water electrolysis as an effective solution. By enabling hydrogen and oxygen production at separate times and locations, this method eliminates the need for expensive membrane separators. Yet, the performance of bipolar electrodes remains a critical factor in ensuring the success of this technology.
Superior Material Performance
The research team’s choice to use cobalt-doped nickel hydroxide is particularly interesting. This combination not only enhances conductivity but also improves electronic storage capacities, addressing previous limitations in electric buffering and charging stability. Cobalt’s role in preventing unwanted oxygen production during hydrogen generation is a game changer, as it directly contributes to the efficiency of the entire process. Furthermore, the incorporation of a one-step electrodeposition method on flexible carbon cloth highlights the innovative approach taken to improve the structural integrity and functionality of the electrodes.
Innovative Catalyst Development
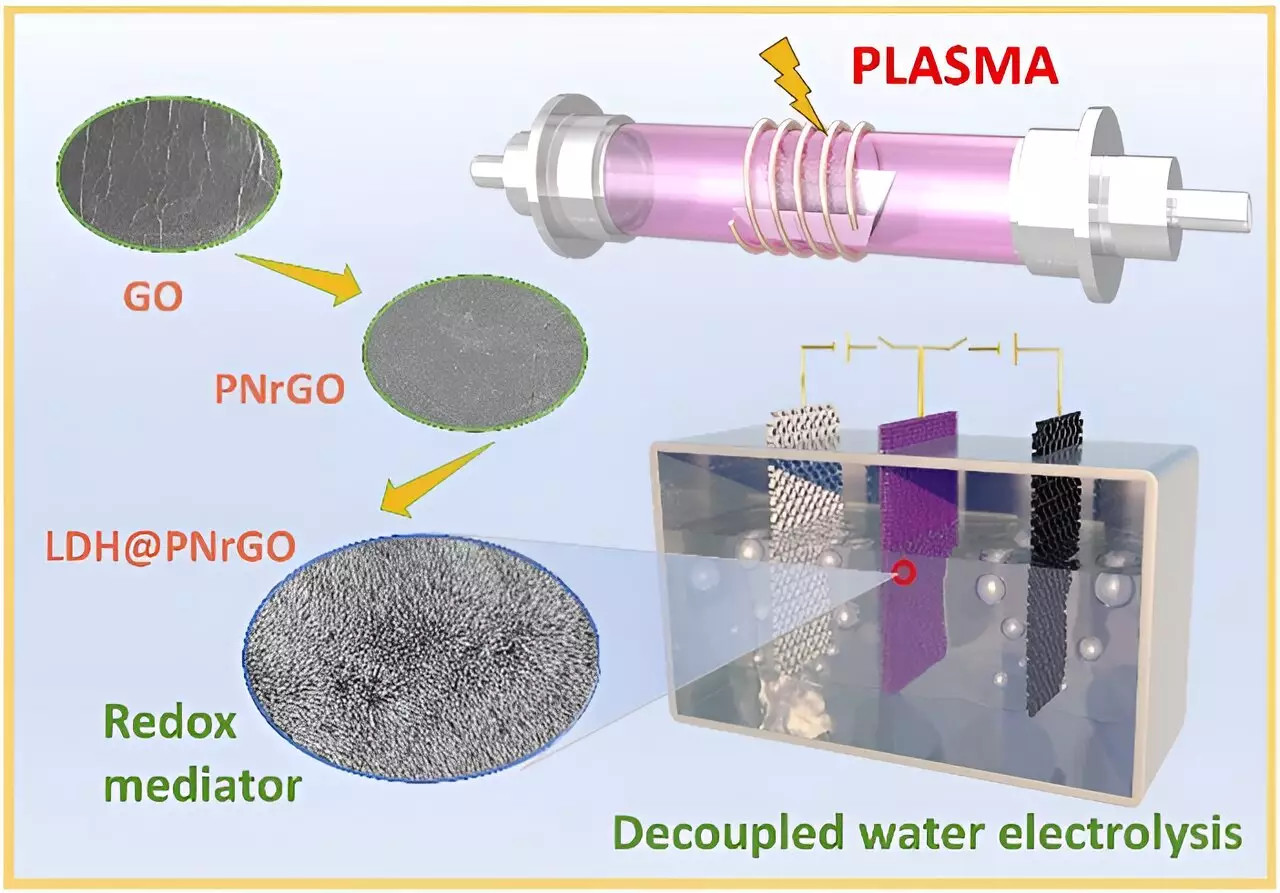
In addition to electrode advancements, the exploration of non-noble metal catalysts, such as molybdenum-doped nickel-cobalt phosphide and plasma-induced iron composite cobalt oxide bifunctional electrodes, underscores a significant shift in material science. These catalysts exhibit exceptional durability and catalytic activity, which are imperative for maintaining high performance in industrial applications. By facilitating a switch of current direction to achieve simultaneous hydrogen and oxygen production at differing times, these innovations allow for considerable reductions in cell voltages and enhanced energy conversion efficiencies.
Pioneering Future Possibilities
The implications of these advancements are profound. The development of layered double hydroxide (LDH) electrodes via non-thermal plasma technology brings forth a new era of improved capacity and conductivity. The potential for widespread application of two-step water electrolysis in sectors such as 5G base stations and data centers illustrates the versatility and promise of this technology. Prof. Chen Changlun’s assertion that their performance indicators align with advanced global benchmarks signifies not just a technical achievement but a significant stride toward industrial applicability.
The race for hydrogen-based energy solutions is intensifying, and the strides made by Prof. Chen’s team represent a critical contribution to sustainability initiatives worldwide. As reliance on renewable energy sources continues to grow, the ability to produce hydrogen efficiently and sustainably could very well define the future of energy consumption.

Leave a Reply