In today’s world, polypropylene is ubiquitous, essential for a wide array of applications from food storage to medical devices. The surge in demand for polypropylene corresponds directly to the increasing necessity for its precursor, propylene. With a constant chase for efficient production methods, a recent development from researchers at the U.S. Department of Energy’s Argonne and Ames National Laboratories is set to transform the manufacturing landscape of propylene. Their study, published in the Journal of the American Chemical Society, highlights a groundbreaking approach to converting propane into propylene, opening new avenues for energy conservation and cost-effective production.
A Breakthrough in Catalytic Conversion
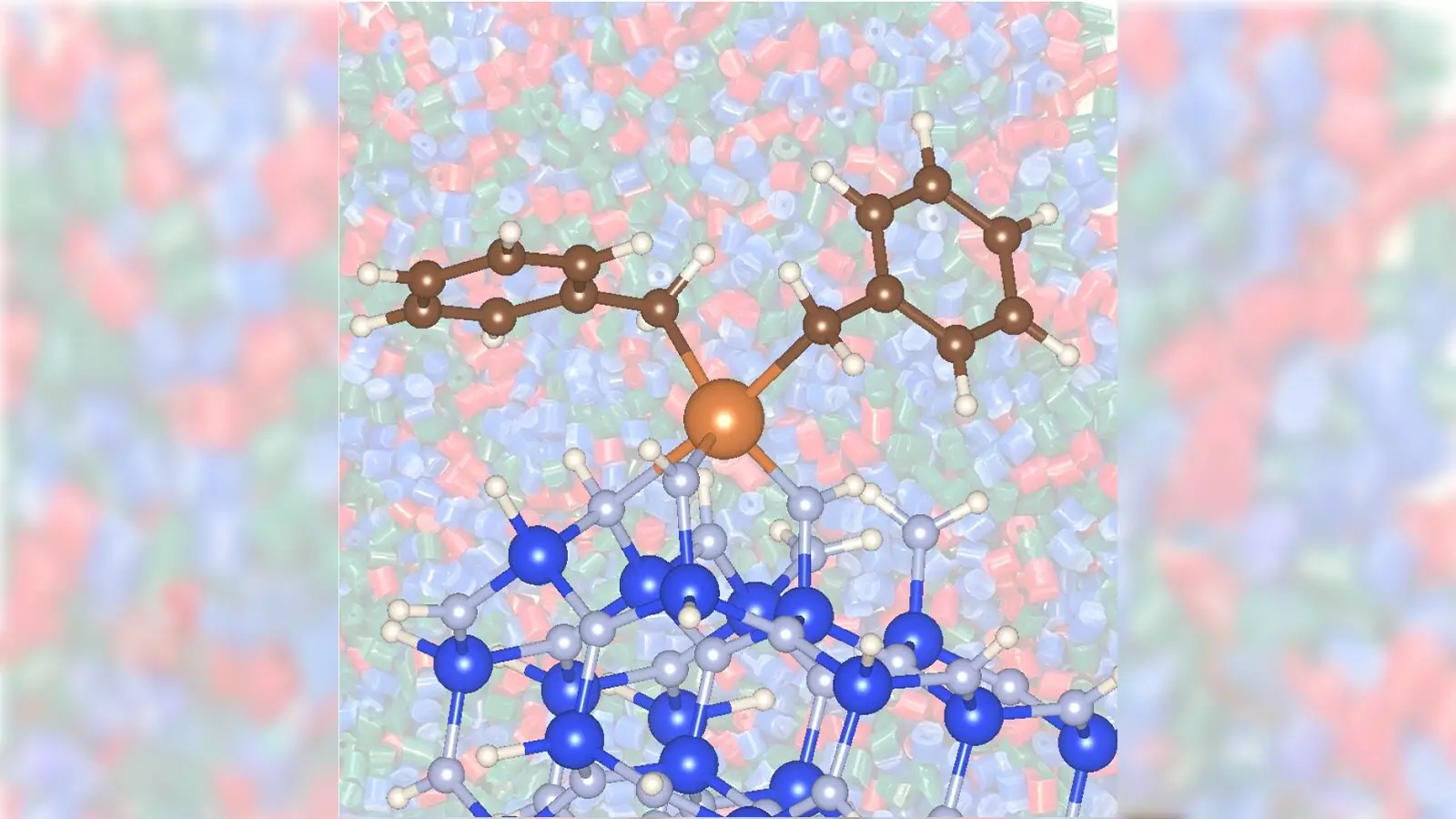
Traditionally, the conversion of propane into propylene involves high operational costs and energy consumption, primarily due to the use of precious metal catalysts such as platinum or chromium. These catalysts, while effective, demand elevated temperatures that contribute excessively to greenhouse gas emissions. However, the collaborative efforts of scientists have unveiled a novel catalytic method using zirconium combined with silicon nitride. This innovation drastically reduces both the energy footprint and the toxicity associated with the standard processes, paving the way for a more sustainable future.
The uniqueness of this discovery lies not only in the material efficiency but also in the acceleration of the catalytic reaction. Researchers assert that this zirconium/silicon nitride combination exhibits a remarkable reactivity, enhancing the conversion rates of propane into propylene while operating at a lower temperature of 842 degrees Fahrenheit, compared to the 1,022 degrees commonly needed today.
Lower Temperatures, Lower Emissions
One of the critical outcomes of this research is the reduced operational temperature, a pivotal factor in diminishing carbon emission levels. Given that carbon dioxide contributes approximately 80% of greenhouse gas emissions in the United States, this advancement holds promise for industries seeking to lower their environmental impact. By mitigating the energetic demands traditionally associated with propylene production, this new method could be a game-changer in both industrial and consumer sectors.
Beyond environmental benefits, the implications for cost reduction in propylene manufacturing are significant. Lower temperatures mean a decrease in energy consumption, translating directly to lower operating costs. This advancement could enhance the competitiveness of American producers in global markets that are increasingly leaning toward sustainability.
The Role of Catalyst Supports in Innovation
A pivotal element of the findings is the role of catalyst supports in bolstering the conversion efficacy of propane. The study emphasizes that the use of silicon nitride as a support material significantly amplifies the catalytic action when paired with zirconium. Unlike conventional silica supports, silicon nitride facilitates reactions more rapidly and efficiently, representing a critical development in catalytic science.
Through meticulous experimentation, the research team discovered that silicon nitride’s surface composition plays a crucial role in promoting catalysis. The intricate interactions at the molecular level uncovered by X-ray absorption spectroscopy provide critical insights into why this innovative combination outperforms traditional catalysts. Understanding these reactions not only benefits propylene conversion but could illuminate pathways for advancing multiple catalytic processes across dozens of chemical manufacturing sectors.
The Power of Collaborative Research
The success of this groundbreaking study can be attributed to the synergy of expertise among researchers from both laboratories. Headed by David Kaphan and Max Delferro, the team drew upon each member’s distinct skill sets to explore the nontraditional catalytic landscape. As Kaphan notes, this collaboration demonstrates the significant potential of combining diverse scientific insights to drive innovation forward.
The contributions of Frédéric Perras, who utilized advanced nuclear magnetic resonance techniques, further stressed the importance of comprehensive characterization methods in understanding complex catalysis. These collaborations exemplify how scientific curiosity and a commitment to environmental stewardship can unify to achieve significant progress—an endeavor that calls for further exploration into how low-cost metals can drive catalytic processes beyond those examined in this study.
The era of energy-efficient propylene production signaled by this research gives hope for future advancements where sustainability and affordability can go hand in hand. As the industry grapples with the challenges of environmental responsibility, the discovery of catalytic alternatives heralds a transformative moment for chemical manufacturing—a step towards a greener, smarter future.

Leave a Reply