The world of microbiology is rife with adaptations that bacteria have developed over millennia, mainly centered around survival. One intriguing aspect of bacterial life is the protective mechanisms employed by pathogenic strains, notably the formation of a capsule. This article delves into the findings of recent research spearheaded by Dr. Timm Fiebig and his team at Hannover Medical School, which sheds light on how these protective barriers are formed and the implications for vaccine development and antibiotic strategies.
Bacterial pathogens have demonstrated a remarkable ability to shield themselves from environmental threats and the host immune response. A key feature of many of these pathogens is their capsule—an outer layer composed of polysaccharides that not only provides physical protection but also conceals the bacterium from the immune system. This camouflage allows them to thrive inside the host, making them a formidable foe in clinical settings.
Capsules serve multiple purposes: they prevent desiccation, shield against physical stress, and function as a critical barrier to immune cell detection and targeting. The ability of a pathogen to build these capsules is directly linked to its virulence. Therefore, understanding the biosynthesis of these structures is paramount for developing drugs that could inhibit their formation, effectively rendering the bacteria vulnerable.
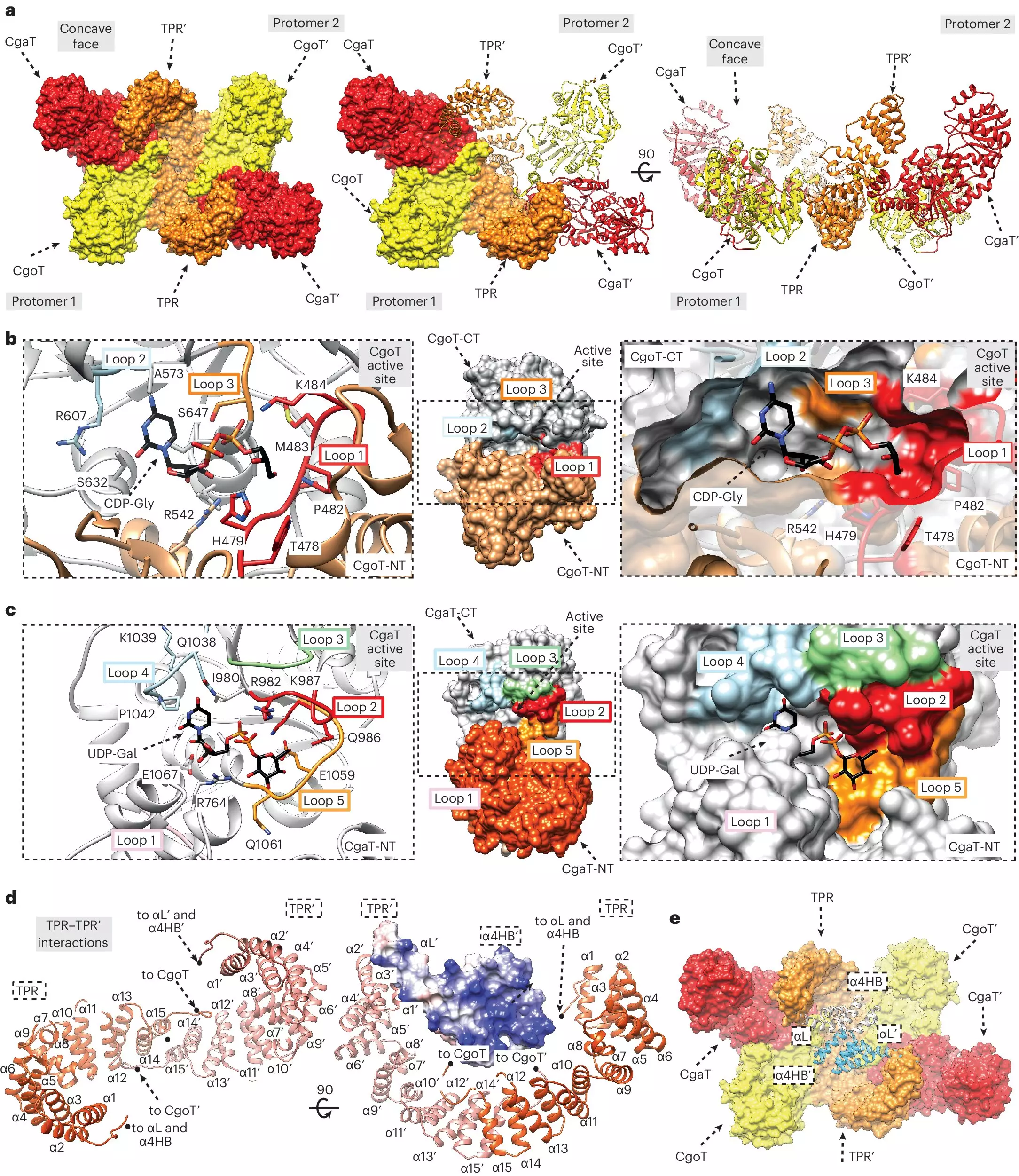
A primary focus of Dr. Fiebig’s research is the identification of enzymes responsible for the biosynthesis of capsule structures. These enzymes, categorized as capsular polymerases and transition transferases, play distinct yet interconnected roles in the assembly of the polysaccharide capsule. Transition transferases are especially intriguing—their function as linkers between the bacterial membrane and the capsule polymers is vital, yet their detailed mechanism remained largely unexplored.
The recent study makes significant strides in characterizing these linkers and their associated enzymes, providing potential targets for therapeutic intervention. The discovery that the genomic locations of transition transferase genes are conserved across various bacterial species hints at a universal mechanism that could be exploited for broad-spectrum antibiotic development.
The research team’s ability to purify the linker alongside the enzymes offers a valuable opportunity to investigate their roles more comprehensively. In their experiments, the researchers successfully mimicked the capsule synthesis process in vitro, thereby allowing for a clearer understanding of how these structures are produced. This foundational knowledge expands our comprehension of bacterial pathogenesis and highlights the intricate biochemical pathways that support their survival.
Dr. Christa Litschko, the study’s first author, emphasizes that contrary to earlier beliefs, the linker exhibits distinctive structural properties compared to the capsule itself. This revelation not only clarifies existing misconceptions in bacterial biochemistry but also pushes the frontier in identifying further enzymes involved in capsule synthesis.
The implications of these findings extend beyond basic research and into the realms of therapeutic application. By targeting the enzymes implicated in capsule formation, scientists can develop novel strategies for vaccines and antimicrobials. Inactivating transition transferases could lead to a state where bacteria are stripped of their protective barrier, making them susceptible to the immune system’s assaults. Thus, the development of inhibitors or drugs targeting these enzymes could enhance the effectiveness of existing antibiotics and facilitate the creation of new vaccines.
Furthermore, this approach is particularly promising given that multiple pathogenic strains share similarities in their capsule formation processes. Such commonalities provide a fertile ground for crafting drugs that are effective against a myriad of bacterial infections, including those responsible for meningitis and urinary tract infections.
The extensive work conducted by Dr. Fiebig and his team opens new avenues for research and exploration within microbiology. By elucidating the structural and functional dynamics of bacterial capsules and their associated enzymes, the study lays a robust foundation for future investigations.
Further research is needed to explore the full spectrum of capsuled pathogens and to expand our knowledge of the enzymatic processes involved. This could ultimately lead to the identification of new therapeutic targets and the development of a new class of antibiotics that could circumvent the growing problem of antibiotic resistance.
The study of bacterial capsules offers a critical perspective on microbial defense mechanisms. By advancing our understanding of these structures, researchers can lay the groundwork for innovative strategies to combat bacterial pathogens, promising hope in the enduring battle against infectious diseases.

Leave a Reply