Hydrogen, a clean and versatile fuel, is increasingly being produced through a process known as photoelectrolysis, which involves the electrolytic splitting of water. One innovative method to facilitate this process is the use of photoelectrochemical cells (PECs). These cells utilize photoelectrodes that capture sunlight and convert it into the electrical energy required for water splitting. This technological advance allows for a sustainable approach to hydrogen production, which presents an alternative to fossil fuels.
The Importance of Efficiency in PECs
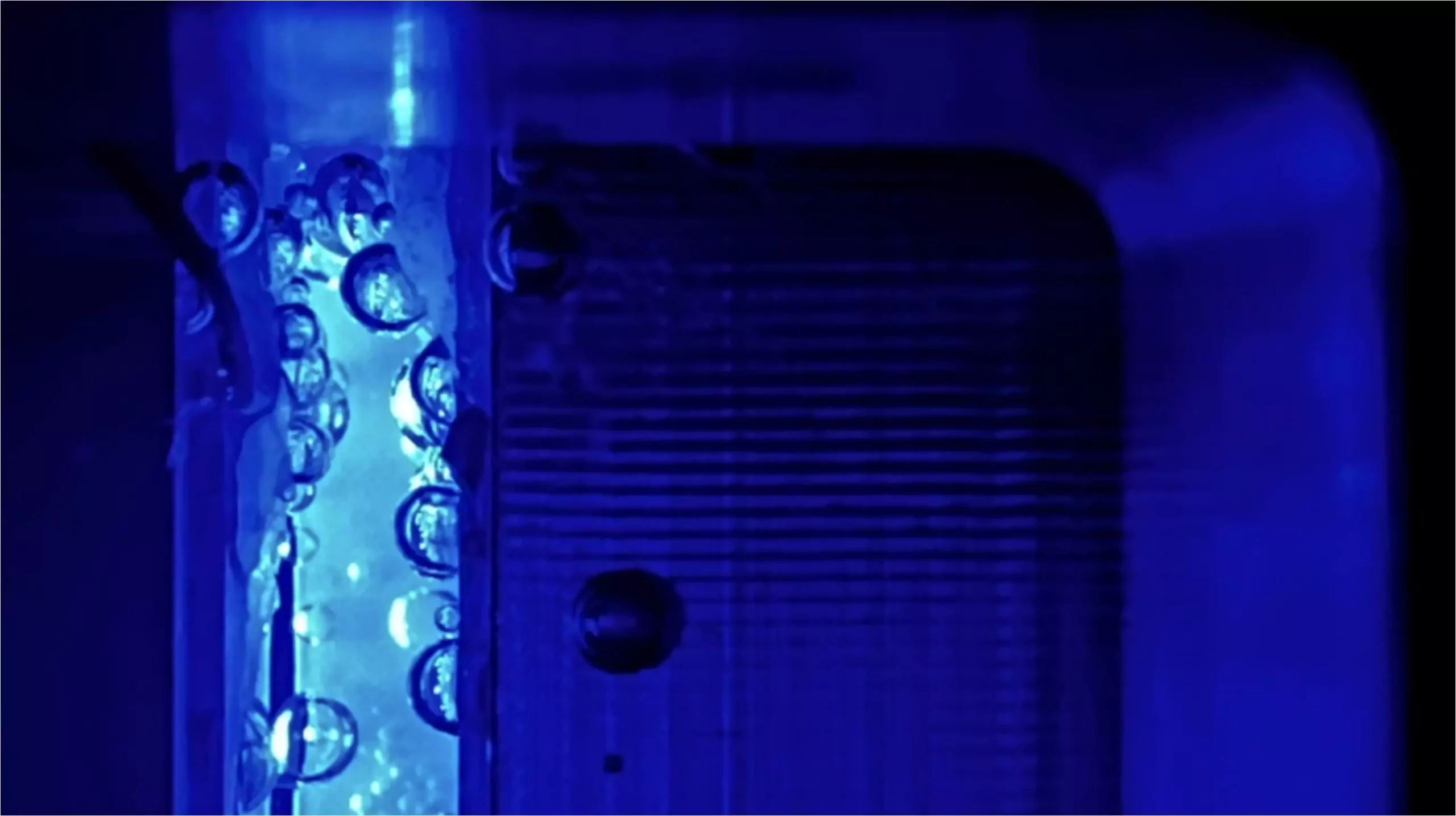
While the theoretical potential for hydrogen production is vast, achieving high efficiency remains a challenge. Recent advancements have led to PECs demonstrating energy conversion efficiencies upwards of 19%, a remarkable achievement that reflects significant progress in the field. However, the quest for optimization doesn’t end here. As efficiencies rise, various phenomena, such as bubble formation during electrolysis, can impede performance. These bubbles not only cause light scattering but can also obstruct the contact between the electrolyte and the electrode, leading to diminished electrochemical activity.
Research Advancements at HZB
Researchers at the Helmholtz Zentrum Berlin (HZB) have undertaken a study that explores the influence of elevated pressure on the efficiency of PECs. Traditionally, PEC systems have been tested at atmospheric pressure. However, the team operated their PEC flow cells at pressures ranging from 1 to 10 bar to investigate how this variable might affect electrolysis. By harnessing gas to pressurize the systems, they aimed to understand the dynamics of bubble formation and the overall effectiveness of hydrogen production under varied conditions.
The Role of the Multiphyysics Model
A critical component of this research involved the creation of a multiphysics model designed to simulate the PEC process. This model serves as a powerful tool for analyzing the interplay of various parameters and predicting how adjusting these can optimize performance. According to Dr. Feng Liang, the lead author of the study, understanding the impact of pressure on bubble size and behavior at the electrodes was central to their investigation.
One of the key findings of the HZB study is that increasing the operating pressure to around 8 bar could significantly mitigate energy losses during the electrolysis process. The research indicates that this adjustment could lead to a 5-10% relative increase in overall efficiency, a substantial gain in the realm of PEC technology. Notably, at this pressure, the scattering of light caused by gas bubbles could be almost entirely eliminated. Furthermore, researchers observed a decrease in unwanted gas transfer, particularly oxygen, to the counter electrode, a common issue that complicates the efficiency of hydrogen production.
While the study underscores the value of elevated pressure, it also reveals a threshold beyond which no additional benefits were recognized. The researchers concluded that an optimal pressure range of 6 to 8 bar would be most effective for PEC electrolyzers.
The significance of these findings extends beyond just water splitting. The multiphysics model created during this research has the potential to inform future advancements in not only electrochemical systems but also photocatalytic devices. As the world steadily pivots towards more sustainable energy solutions, optimizing hydrogen production methods will play a critical role in meeting global energy demands while reducing reliance on fossil fuels.
The innovative research conducted by the HZB team highlights a promising direction in the field of hydrogen production via photoelectrochemical cells. By employing elevated pressure as a means to enhance efficiency, they may have set the stage for further breakthroughs in energy conversion technologies. As we continue to explore sustainable energy pathways, studies like these remind us of the importance of interdisciplinary approaches and innovative thinking in tackling the challenges of our time. The quest for efficient and cost-effective hydrogen production is not merely a scientific pursuit; it is an essential step toward a greener future.

Leave a Reply