The quest to enhance oxidation potentials has brought forth significant advancements in the field of molecular and coordination chemistry. A pivotal study led by Professor Ingo Krossing at the University of Freiburg has unveiled a groundbreaking technique that increases the oxidation potential of positive ions, surpassing traditional limits. This article will delve into the ramifications of this research, the methodologies employed, and the broader implications for chemical processes.
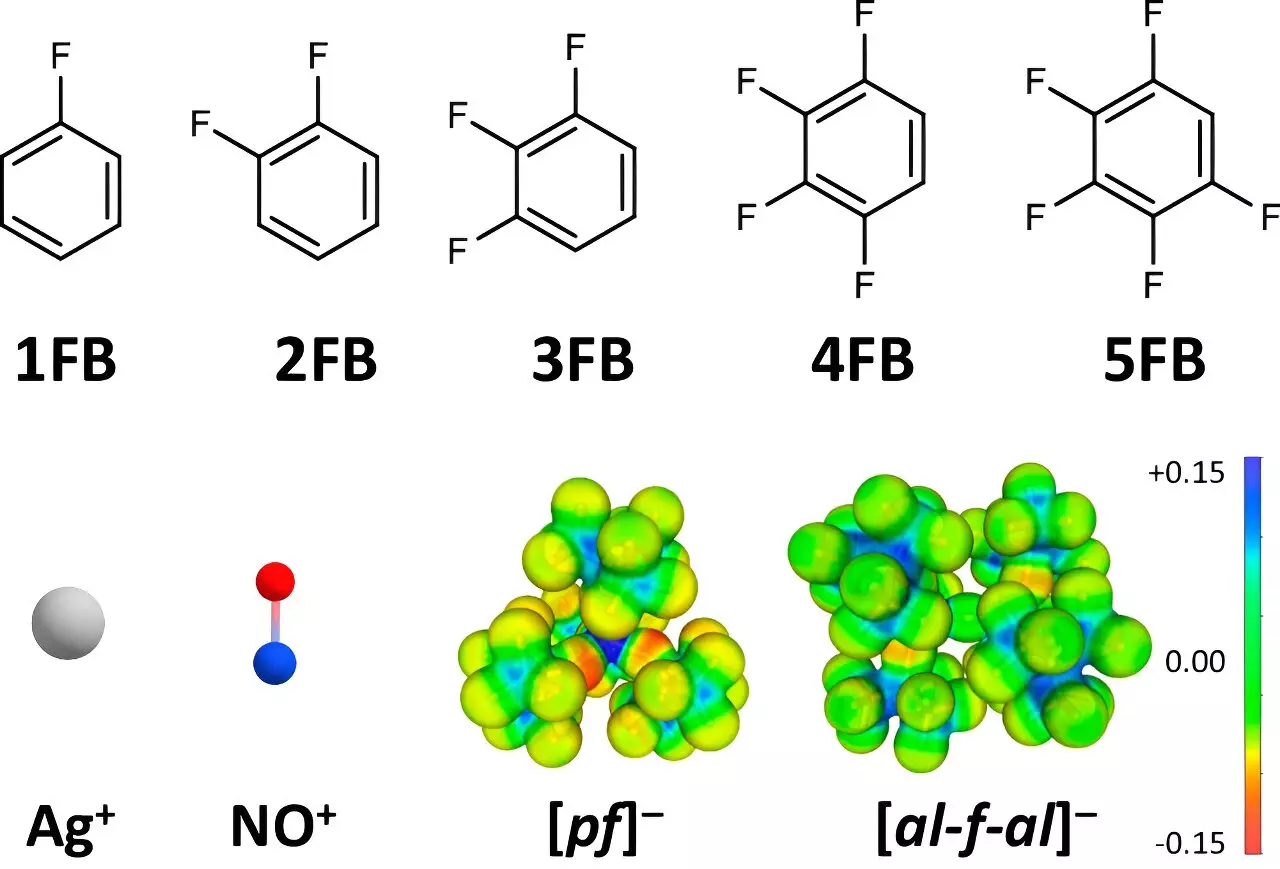
Oxidation potential is a critical parameter in electrochemistry, enabling various reactions essential for materials science, electrochemical cells, and catalysis. Traditionally, positive ions such as Ag+ and NO+ have exhibited limited oxidation potentials, typically reaching values between +0.65 and +1.0 V against a reference electrode, Fc+/0. The innovative approach taken by Krossing and his team, however, showcases remarkable results with potentials of up to +1.52 V being achieved. This advancement not only expands the theoretical boundaries of oxidation potential but also opens the door to practical applications previously deemed unfeasible.
The cornerstone of this research lies in the use of weakly coordinating anions (WCAs) and solvents. The team’s focus on strategically substituting fluorinated benzene derivatives highlights a pivotal shift in the selection of solvent environments. Common solvents, such as dichloromethane or acetone, exhibit strong interactions with positive ions, thereby diminishing their oxidation potential. Conversely, the unique properties of fluorinated benzene derivatives allow for reduced interactions, preserving the integrity of the positive ions and enhancing their oxidation abilities.
Dr. Johannes Hunger from the Max Planck Institute for Polymer Research contributed pivotal data regarding the dielectric constants of these solvent molecules. This analysis illustrated that fluorinated options not only reduce interaction strength but also maintain higher dielectric constants, further promoting the necessary conditions for enhanced oxidation.
The methodological framework of this research integrated electrochemical measurements with structural analysis via single-crystal X-ray diffraction. Such multidimensional scrutiny allowed researchers to ascertain how the structural arrangements of compounds correlate with their electrochemical behavior. Dr. Malte Sellin, a co-author of the study, emphasized that greater degrees of fluorination directly correlate with weaker interactions between the solvent and the positive ions, leading to the astonishing oxidation potentials observed.
Understanding these interactions unlocks new pathways for chemical reactions that were previously underexplored. By minimizing interference from the solvent, the researchers have created an environment where the chemical characteristics of Ag+ and NO+ can be fully utilized.
The ramifications of Krossing’s work extend beyond the confines of academic research. By achieving higher oxidation potentials, this research paves the way for innovative applications in electrocatalysis, redox shuttles, and mediators. The implications are especially relevant in fields targeting renewable energy solutions, advanced materials, and efficient chemical synthesis. As scientists seek methods to harness these conditions for reactions that are traditionally considered challenging or impossible, the potential for significant breakthroughs in catalysis and energy storage becomes apparent.
Moreover, this work could lead to novel chemical studies that explore the intricate behaviors and reactions of previously unexamined systems, fueling the development of new materials or chemical pathways that can respond to contemporary industrial challenges.
The advancements made by the research team at the University of Freiburg mark a significant milestone in coordination chemistry and electrochemistry. By ingeniously manipulating solvent interactions through the use of weakly coordinating solvents and strategically fluorinated compounds, this study not only illustrates the ability to enhance oxidation potentials but also highlights a transformative approach to future chemical research. The implications of this work resonate through both academic circles and practical applications, potentially revolutionizing the way we understand and utilize oxidation in the chemical sciences.

Leave a Reply