The dynamic landscape of modern chemistry often grapples with the inherent challenges posed by traditional reaction mediums, especially the pervasive use of toxic organic solvents. A critical concern arises from the fact that these solvents contribute to over 80% of the waste generated in chemical processes, not to mention the risk factors associated with improper disposal. In an impressive stride toward greater sustainability, a research team from the Indian Institute of Science (IISc) has developed a groundbreaking solution that utilizes agricultural waste to catalyze important chemical reactions, heralding a new era in sustainable chemistry.
The ambitious initiative led by researchers at the Department of Inorganic and Physical Chemistry demonstrates a pivotal shift from reliance on harmful solvents. Their innovative surfactant, named CNSL-1000-M, is synthesized from cashew nut shell liquid (CNSL)—an agricultural byproduct that emerges during the cashew roasting process. This move reflects not only a commitment to greener practices but also addresses the economic viability of utilizing readily available raw materials. As India stands as the world’s second-largest producer of cashew nuts, accessing CNSL translates to lower costs and reduced environmental impact.
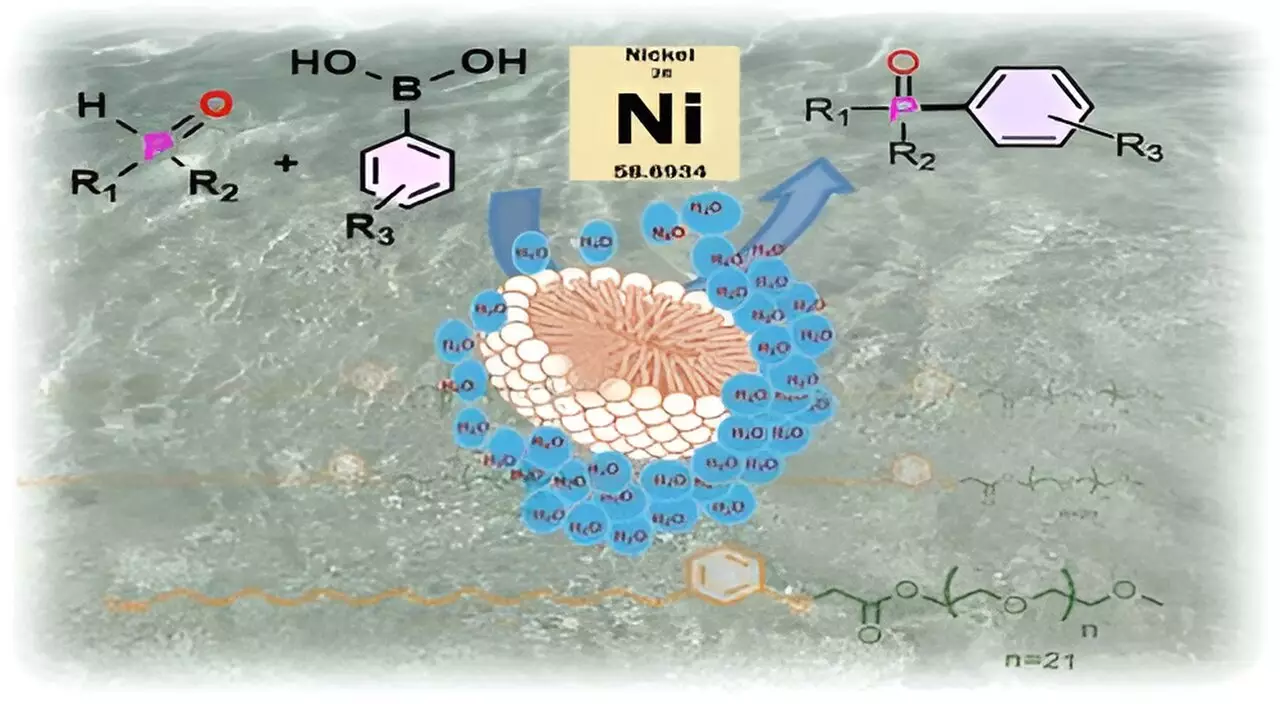
By paving the way for micellar catalysis, these researchers challenge the orthodoxy of conventional chemistry. Surfactants, by their nature, possess both hydrophilic and hydrophobic traits, allowing them to create micelles in aqueous environments. When introduced to water, CNSL-1000-M organizes itself into micelles that effectively offer pockets of chemical protection. This methodology leverages the surfactant’s unique structure, allowing substrates to be shielded from the deleterious effects of water—a crucial factor for many sensitive catalysts.
The scientific principles underpinning micellar catalysis draw striking parallels to natural biological processes. By resembling the action of enzymes that contain hydrophobic pockets, the CNSL-1000-M surfactant fosters an innovative approach to reaction conditions. Imagine a football bobbing atop a body of water; as long as the ball remains sealed, it keeps the water out. Similarly, substrates encased within the micelles can undergo reactions in a sheltered environment that is otherwise hostile.
A significant example of the efficacy of this approach is showcased in the successful catalysis of carbon-phosphorus bond formations—an essential step in synthesizing several key compounds, including the anti-cancer drug Brigatinib. The data emphasizes an impressive efficiency of the CNSL-1000-M surfactant, with an 80% increase in product yield in comparison to traditional processes utilizing organic solvents. Furthermore, researchers noted a remarkable 30% improvement in yield over existing surfactants when utilizing aqueous protocols.
The implications of this research extend far beyond a mere laboratory curiosity; they paint a promising picture for the future of industrial chemistry. The ability to use CNSL-1000-M not only replaces expensive precious metal catalysts like palladium, allowing for the transition to more affordable nickel complexes, but also enables operations at lower temperatures—further enhancing both efficiency and sustainability in chemical manufacturing processes.
By actively engaging with industries, this research team seeks to facilitate a meaningful transition towards a sustainable and green alternative to toxic solvents. The potential impacts of adopting micellar catalysis across various sectors not only position industries towards compliance with environmental standards but also showcase the economic benefits derived from reduced waste and resource consumption.
The strides made by researchers at IISc exemplify a crucial shift in how the chemical industry approaches sustainability. Through innovation rooted in agricultural waste, the development of CNSL-1000-M surfactant marks a significant turning point in micellar catalysis as a powerful alternative to traditional organic solvents. The merging of chemistry and sustainability sets a promising precedent as the scientific community continues to grapple with the dual challenges of efficiency and environmental stewardship. As this technology is further refined and industrially implemented, it holds the potential to revolutionize practices within the field, driving a sustainable future for chemical synthesis.

Leave a Reply