The world of scents has enchanted humanity since antiquity, becoming an integral part of cultural identity and personal expression. The allure of perfumes stretches back to ancient civilizations, where the essence of aromatic compounds was highly cherished, not just for their olfactory benefits but also for their significance as indicators of health and vitality. As societies evolved, so too did the methods of fragrance extraction and synthesis. Among the myriad of compounds, ambrox stands out for its unique and captivating aroma, derived primarily from the elusive ambergris, a substance produced in the intestines of sperm whales. Despite its exotic origins, the complexities surrounding ambrox production have long posed ethical and environmental dilemmas.
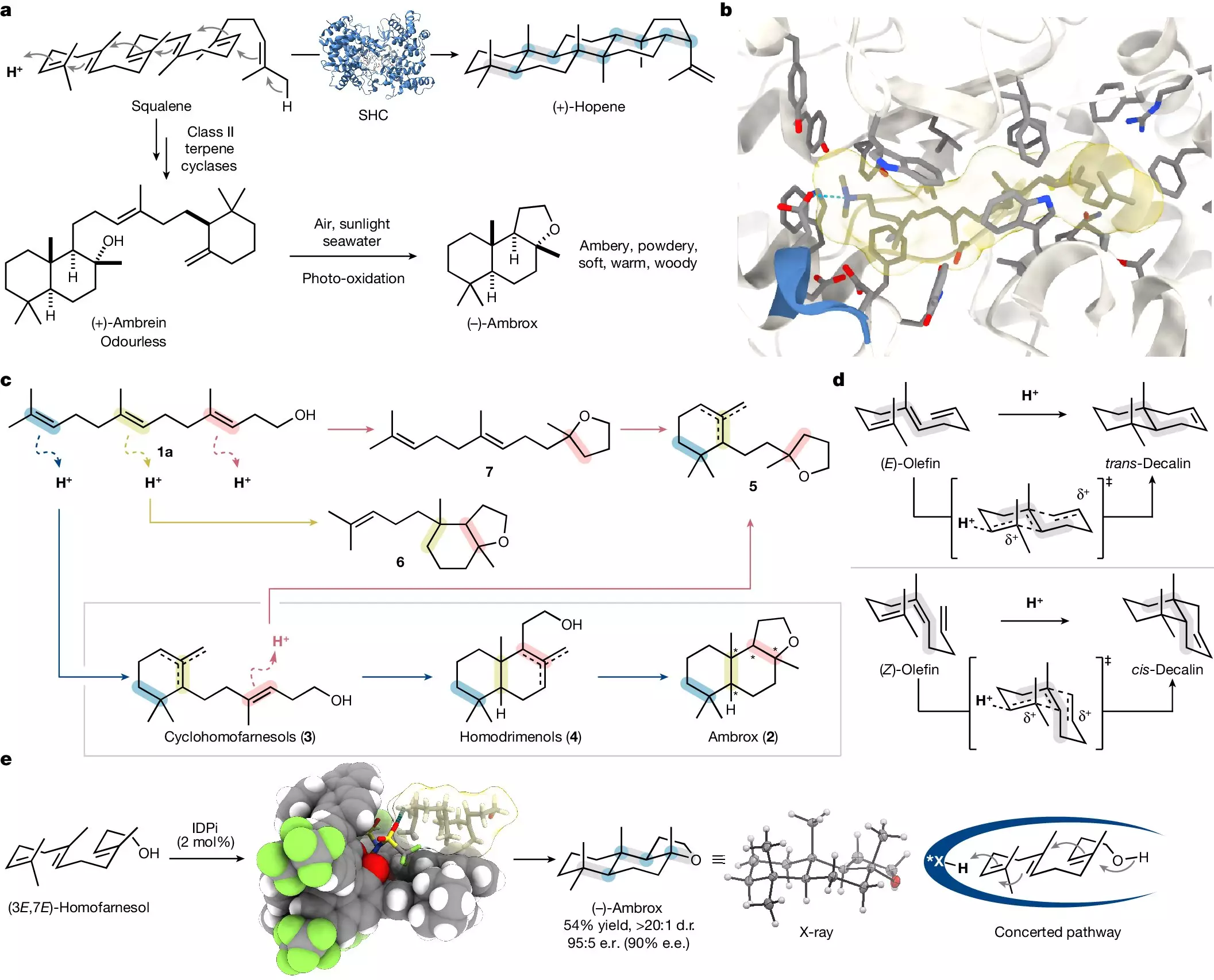
For centuries, ambrox has been in high demand within the fragrance industry, resulting in extensive harvesting of ambergris. However, the unpredictable availability of this natural resource has led researchers and perfumers alike to seek more sustainable alternatives. Surprisingly, ambrox is a chiral molecule, capable of existing in multiple forms, yet only one of its sixteen variants offers the highly sought-after scent profile. This revelation complicates the production process, necessitating advanced methods to selectively synthesize the correct isomer without resulting in wasteful byproducts.
Fortunately, recent advancements in synthetic chemistry have begun to shift the landscape. Drawing inspiration from nature, scientists can now harness plants like the clary sage, which produces sclareol, a precursor to ambrox. This process, while more sustainable than sourcing ambergris, still depends on variable agricultural output and remains complex, requiring multiple steps and substantial labor.
A turning point in ambrox synthesis arrived through the groundbreaking work of Professor Benjamin List and his team at the Max Planck Institute for Coal Research. They developed a catalytic asymmetric polyene cyclization method that revolutionizes how complex chiral molecules can be produced in laboratory settings. Their findings, published in the prestigious journal Nature, illuminate a new pathway for chemists, marrying the simplicity of starting materials with the intricate structures found in nature.
The method orchestrates the transformation of homofarnesol—a compound abundant in many plant species—into the elusive ambrox in just a single step. Mathias Turberg, a doctoral candidate in List’s lab, emphasizes the significance of this approach: “We aimed to simplify the synthesis of complex molecules while ensuring high selectivity in the final product.” The pivotal moment comes from mimicking the natural process that enzymes utilize to guide polyene compounds to fold into specific shapes, facilitating the emergence of the desired isomer.
At the heart of List’s novel method is a tailored catalyst and a specialized fluorinated solvent, which together create an ideal environment for the reaction to unfold. Dr. Na Luo, another key contributor to the research, reveals that this careful orchestration not only enhances selectivity but also significantly accelerates the reaction timeline. “What typically takes several days can now be achieved overnight, all while maintaining mild reaction conditions,” she explains.
Moreover, the methodology brings sustainable advantages, as both the catalyst and solvent can be recovered and reused, minimizing waste and encouraging industrial scalability. This dual benefit positions the new synthesis as a promising contender for green chemistry applications, promoting a synergy between scientific innovation and environmental conscience.
The implications of this synthesis extend beyond the realm of perfumery. The ability to efficiently create complex chiral molecules lays the groundwork for enhanced production methods in various industries, including pharmaceuticals and materials science. By learning from nature’s underlying principles, scientists can develop products that are not only effective but also environmentally sustainable.
As the fragrance industry continues to grapple with sustainability challenges, the work of Prof. List and his team symbolizes hope for the future. With the potential to pivot from resource-intensive methods to laboratory-based synthesis, ambrox may well emerge as a case study in how chemistry can adapt and thrive in a world increasingly cognizant of its ecological footprints. Through relentless innovation, the relationship between humanity and the environment can evolve, leading to a new era of chemistry that upholds the values of both beauty and sustainability.

Leave a Reply