Ammonia stands as a cornerstone in food production and various industrial applications, with a staggering global market size of around 175 million metric tons, translating to a market value estimated at $67 billion. Its prominence can be attributed to its applications in fertilizers, chemical synthesis, and its potential as a high-energy-density carrier in the burgeoning hydrogen economy. However, conventional ammonia synthesis methods, primarily the Haber-Bosch process, pose significant environmental challenges. This energy-intensive method contributes to considerable CO2 emissions, emphasizing the urgent need for innovative and sustainable alternatives.
Recent advancements led by a research group at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR), under the leadership of Hao Li, have turned the spotlight on the electrochemical conversion of nitrates (NO3–) to ammonia (NH3). A study published in *Advanced Science* on August 9, 2024, presents this promising approach, suggesting that it could revamp industrial ammonia production and mitigate many existing environmental concerns. Unlike the nitrogen reduction reaction (NRR) that involves breaking the robust N=N triple bond found in nitrogen (N2), nitrate reduction (NO3RR) emerges as a superior alternative due to its lower dissociation energy and higher water solubility. This facilitates a more efficient pathway for ammonia synthesis while simultaneously addressing the ecological issue of nitrate accumulation in water systems.
Innovative Catalysts and Their Performance
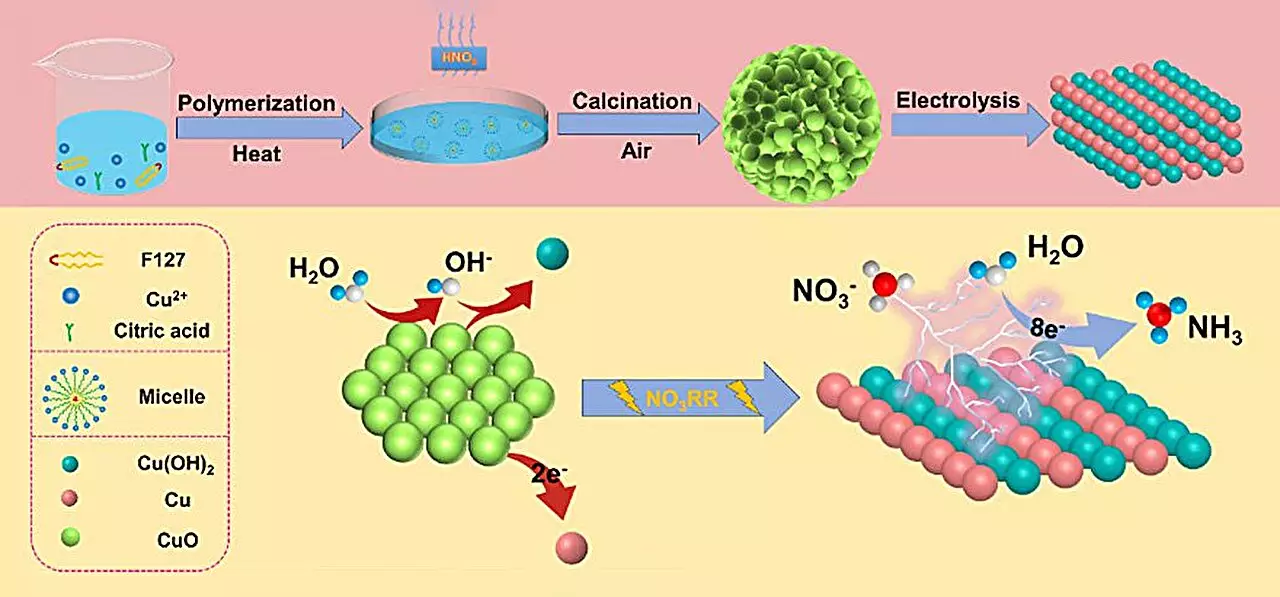
The research team focused on developing a novel spherical copper (II) oxide (CuO) catalyst, which is characterized by small particles featuring oxygen-rich vacancies. This cutting-edge catalyst showed extraordinary performance in ammonia production, achieving a yield of 15.53 mg h-1 mgcat-1 and a remarkable Faraday efficiency of 90.69% under neutral electrolyte conditions at -0.80 V (versus a reversible hydrogen electrode). The underlying reason for this high catalytic activity can be attributed to structural and phase changes that occur during the electrochemical reduction process.
Qiuling Jiang, a co-author and joint Ph.D. student at WPI-AIMR, emphasized the significance of the transformation from CuO to Cu/Cu(OH)2 during the reaction. This phase transition enhances the catalyst’s overall performance by increasing the number of active catalytic sites and improving electron transfer at the electrode interface. Such advancements not only promise improved efficiency but also unlock new pathways for ammonia synthesis.
To comprehend the mechanisms behind this enhanced catalytic activity, the researchers employed density functional theory (DFT) calculations. These computations illuminated how the formation of Cu(OH)2 lowers the energy barriers associated with nitrate adsorption, rendering the process energetically feasible. Additionally, the Cu(OH)2 phase acts to deter the competing hydrogen evolution reaction, while the presence of Cu (111) crystal facets boosts the hydrogenation process, further refining overall yield.
Li elaborates on the implications of this research, indicating that advancements in the design of copper-based catalysts for electrocatalytic ammonia production could lead to broader applications in sustainable industrial practices. By controlling reaction conditions and monitoring phase transitions, the catalysts’ performance can be significantly optimized, paving the way for the implementation of more efficient ammonia synthesis methods.
Looking to the future, the research team aims to delve deeper into the factors influencing phase transitions, with an eye toward enhancing the catalysts’ stability, activity, and selectivity. The ultimate goal is to realize sustainable ammonia production that not only meets industrial demands but also supports environmental stewardship.
The study spearheaded by Hao Li and his team at Tohoku University represents a significant leap forward in the quest for efficient ammonia synthesis. Shifting the focus from traditional methodologies to innovative electrochemical processes could herald a new era for the ammonia industry—one that emphasizes sustainability and environmental responsibility without compromising efficiency.

Leave a Reply