Recent research from the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig has opened a new chapter in atmospheric chemistry with the revelation of sulfurous acid (H2SO3) existing under atmospheric conditions in gas phase. This groundbreaking discovery challenges long-standing beliefs about the compound’s existence and stability, redefining its significance within the sulfur cycle of our atmosphere. Published in the prestigious journal Angewandte Chemie, the study illuminates the complexity of atmospheric chemistry and the relevance of previously elusive compounds.
Sulfurous acid, in contrast to the more commonly recognized sulfuric acid (H2SO4), had been considered an enigma in atmospheric science. While textbooks generally describe H2SO3’s formation in aqueous solutions of sulfur dioxide (SO2), its existence as a standalone molecule has remained speculative at best. Despite extensive experimental attempts to detect H2SO3 in sulfate solutions using various spectroscopic techniques, researchers have encountered significant obstacles, primarily yielding only its related ionic forms: bisulfite (HSO3−) and sulfite (SO3²⁻).
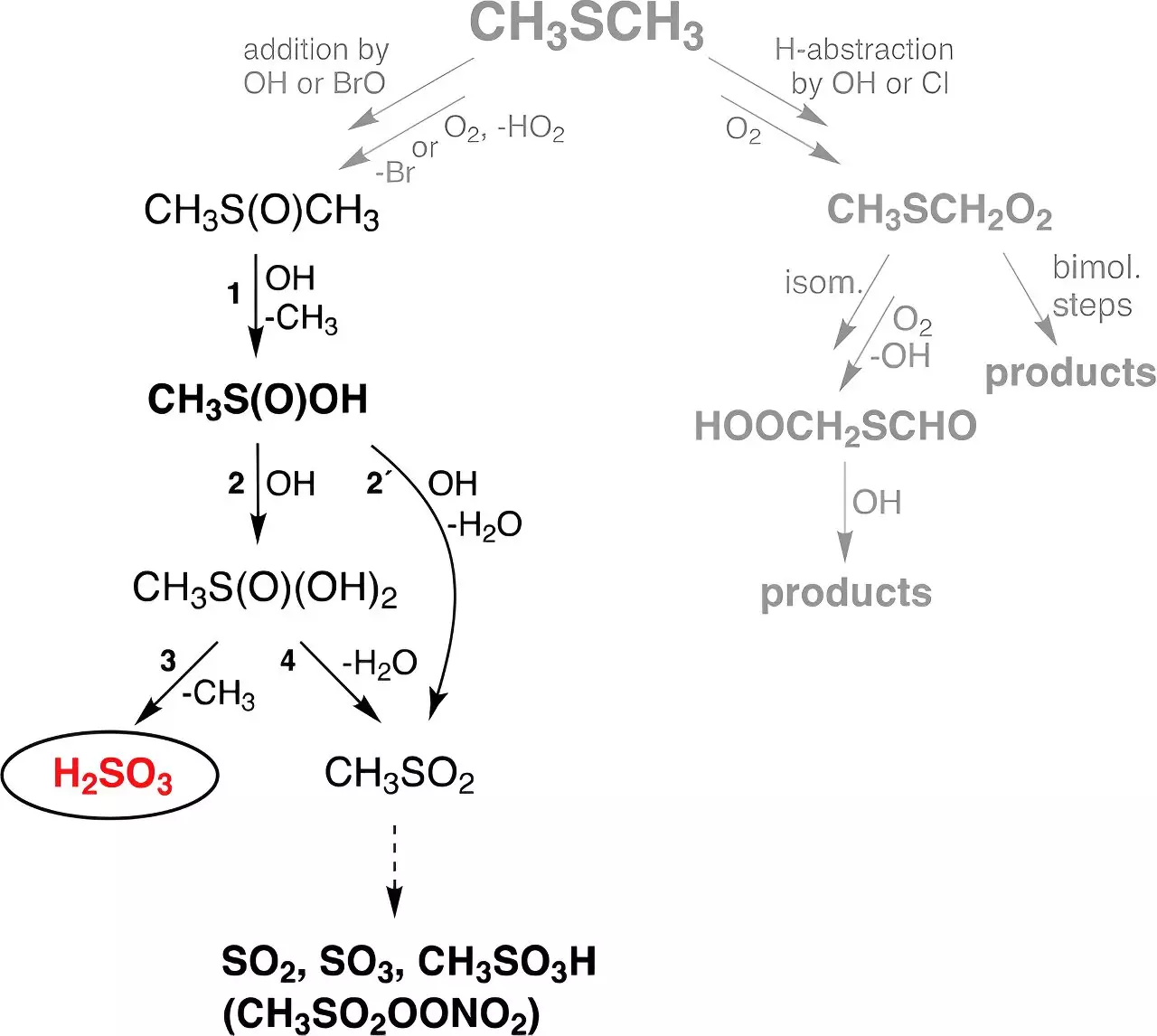
The only experimental identification of H2SO3 emerged back in 1988 when Helmut Schwarz’s team at TU Berlin succeeded in its detection via in-situ generation within a mass spectrometer. Their findings indicated that H2SO3 had an extremely brief lifespan under vacuum conditions, lasting approximately only 10 microseconds. Predominant theoretical models proposed that the atmospheric gas-phase reaction of hydroxyl (OH) radicals—originating from water vapor and ozone under ultraviolet (UV) radiation—could lead to H2SO3 formation when interacting with dimethyl sulfide (DMS). As a crucial biogenic sulfur compound produced primarily in marine environments, DMS significantly impacts atmospheric chemistry, contributing about 30 million tons of sulfur annually.
Building on these foundational theories, the team at TROPOS explored the viable pathways that might enable the formation of H2SO3 from DMS. Their experimental work employed flow reactors designed to simulate atmospheric conditions, leading to the significant finding that H2SO3 can exist in a stable gas phase for approximately 30 seconds, regardless of the ambient humidity. This pivotal discovery prompts a reevaluation of the potential role H2SO3 may occupy within atmospheric processes, as its longevity raises possibilities for influence on broader chemical reactions.
The TROPOS team, led by Dr. Torsten Berndt, successfully incorporated their experimental insights into a global chemistry-climate model. The ensuing simulations revealed that roughly 8 million tons of H2SO3 are now believed to form annually across the globe. Intriguingly, this newly established reaction pathway yields significantly more H2SO3—approximately 200 times greater—than the previously established formation routes of sulfuric acid from DMS in an atmospheric context.
This extensive output underscores the profound implications for scientists seeking to understand the complexities of the atmospheric sulfur cycle better. As emphasized by Dr. Andreas Tilgner and Dr. Erik Hoffmann, who contributed to the global modeling aspect of the study, these findings offer vital insights that could alter our understanding of sulfur dynamics in the atmosphere.
Future Directions: Unanswered Questions
Despite the remarkable nature of these discoveries, many questions remain unanswered. The apparent stability of H2SO3 in gas form leaves uncertainties regarding its permanence and reactivity within the atmosphere. While current insights suggest stability for an interval of time, its interactions with trace atmospheric gases, including water vapor, remain inadequately understood. Dr. Berndt points out that in-depth research utilizing more refined experimental techniques is crucial to elucidate H2SO3’s full significance and properties.
This research serves as a compelling reminder of how collaboration between advanced experimental design and cutting-edge detection technologies can unveil phenomena previously considered elusive. The mass spectrometer used during the study illustrates this capacity, with a remarkable detection limit able to identify specific molecules within an enormous concentration of other atoms—up to 1 quadrillion molecules.
The detection of sulfurous acid marks an exciting milestone in atmospheric chemistry, prompting scientists to rethink how they view complex biochemical processes. As we strive to comprehend the intricate interactions within the atmosphere, further inquiries into the implications of H2SO3’s formation could provide critical insights into the stability, reactivity, and overall impact on environmental science and climate models. Continuous advancements in detection capabilities will undoubtedly offer deeper revelations into the ever-fascinating world of atmospheric chemistry, enriching our understanding of the delicate balance that governs our planet’s climate and atmosphere.

Leave a Reply