Dinitrogen, or N2, constitutes approximately 80% of the Earth’s atmosphere, yet leveraging this abundant resource in chemical synthesis has been a formidable challenge for chemists. The inherent stability of the dinitrogen molecule, characterized by its strong triple bond, renders it particularly unreactive. This poses significant difficulties when attempting to incorporate nitrogen into complex molecules, such as pharmaceuticals and polymers, which are vital for numerous industrial applications. Current methodologies primarily utilize the Haber–Bosch process to convert dinitrogen into ammonia, a prerequisite for synthesizing nitrogen-containing compounds. However, this traditional route is energy-intensive and inefficient, prompting researchers to seek more sustainable alternatives.
The conventional synthesis of alkyl amines—a type of nitrogen-containing compound—inevitably involves multiple steps that complicate the process. First, the dinitrogen gas must be reduced to ammonia, followed by the further transformation of alkenes into more reactive forms, such as alcohols or carboxylic acids. This not only elongates the reaction time but also increases the energy requirements, leading to a significant carbon footprint. Indeed, the current reliance on fossil fuel-based energy for these transformations raises concerns regarding sustainability. The need for efficient, direct pathways to utilize dinitrogen, along with commercially available alkenes, has never been more pressing.
RIKEN’s Groundbreaking Research
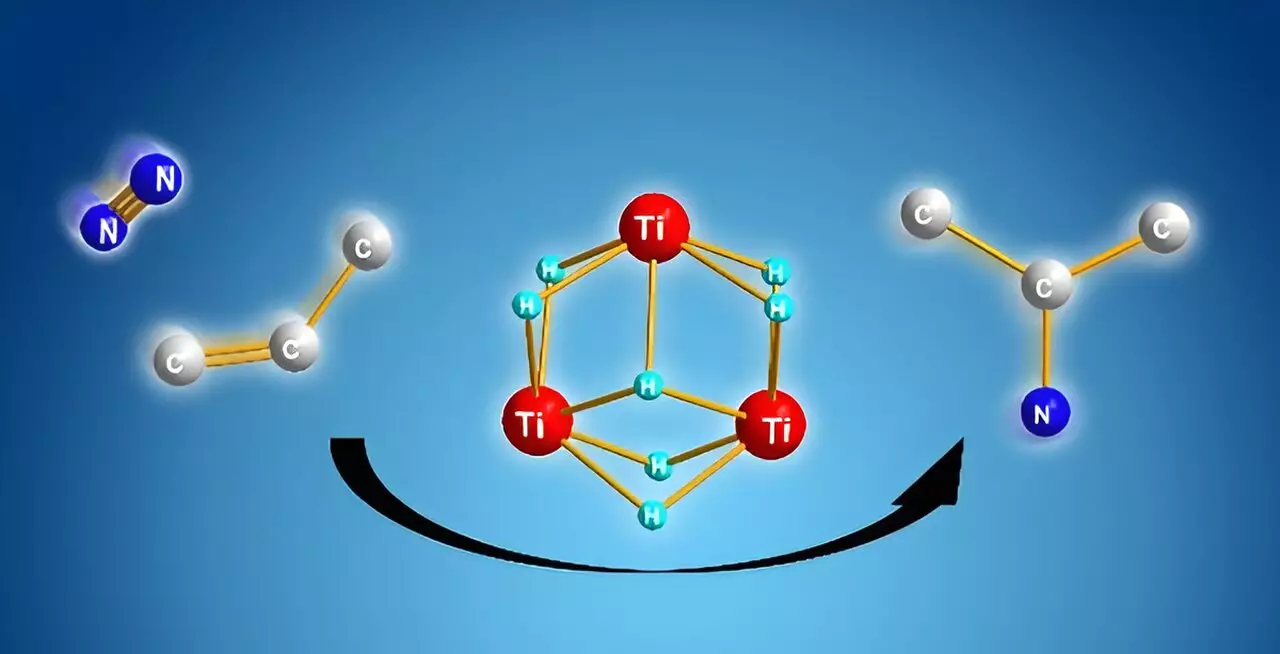
Amidst these challenges, researchers at RIKEN, led by Takanori Shima, have made groundbreaking strides in the use of dinitrogen for the synthesis of alkyl amines. Their innovative approach hinges on the use of titanium polyhydrides—complexes made of titanium and hydrogen that exhibit exceptionally high reactivity towards stable molecules. Prior studies indicated that these complexes could facilitate interactions with inert substrates such as dinitrogen and benzene. However, the discovery that multiple titanium–hydride units can work in concert to activate dinitrogen and promote the synthesis of nitrogen-carbon bonds is a significant advancement.
The team’s research suggests that titanium polyhydride not only activates alkenes but also maintains many titanium-hydride units post-reaction, creating a unique environment for nitrogen functionality. The cooperative cleavage of the dinitrogen molecule by these active sites significantly alters the traditional view of nitrogen chemistry, providing a new way to build valuable compounds efficiently.
Mechanisms of the Reaction
A detailed computational analysis revealed the reaction’s underlying mechanics, emphasizing that the formation of nitrogen-carbon bonds is energetically favored over other potential pathways, such as the generation of nitrogen-hydrogen or carbon-hydrogen bonds. The titanium framework offers an ideal setting for these reactions to occur, streamlining the process dramatically. By activating both dinitrogen and alkenes, the team found efficient pathways to synthesize alkyl amines, suggesting a novel method that overcomes the limitations of previous techniques.
As environmental concerns continue to escalate, the shift towards more sustainable chemical processes is critical. Shima and his colleagues are now exploring ways to further refine this innovative technique, potentially converting it into a catalytic process that would enable broader application in industrial settings. The ability to utilize dinitrogen directly in synthesis is not merely an enhancement to existing methods, but a beacon of hope for the development of greener chemistry.
The implications of this research extend far beyond academic interest, promising to revolutionize how essential compounds are synthesized in diverse fields, from pharmaceuticals to agriculture. As chemists push the frontiers of science towards sustainability, this discovery could pave the way for a paradigm shift, making dinitrogen not just a bystander in chemical reactions, but a key player in the quest for efficient and environmentally friendly synthesis.

Leave a Reply