Hydrogen has long been touted as a potential game-changer in the quest for sustainable energy. Its clean combustion produces only water vapor, making it an appealing alternative to fossil fuels. However, the widespread adoption of hydrogen as a viable fuel source has been hindered primarily by its storage challenges. Unlike gasoline, hydrogen occupies a significantly larger volume, making efficient storage solutions essential for its practical use. Researchers across the globe have sought innovative methods to compress hydrogen without adding weight or complexity, leading to a focus on supramolecular materials that can effectively address these issues.
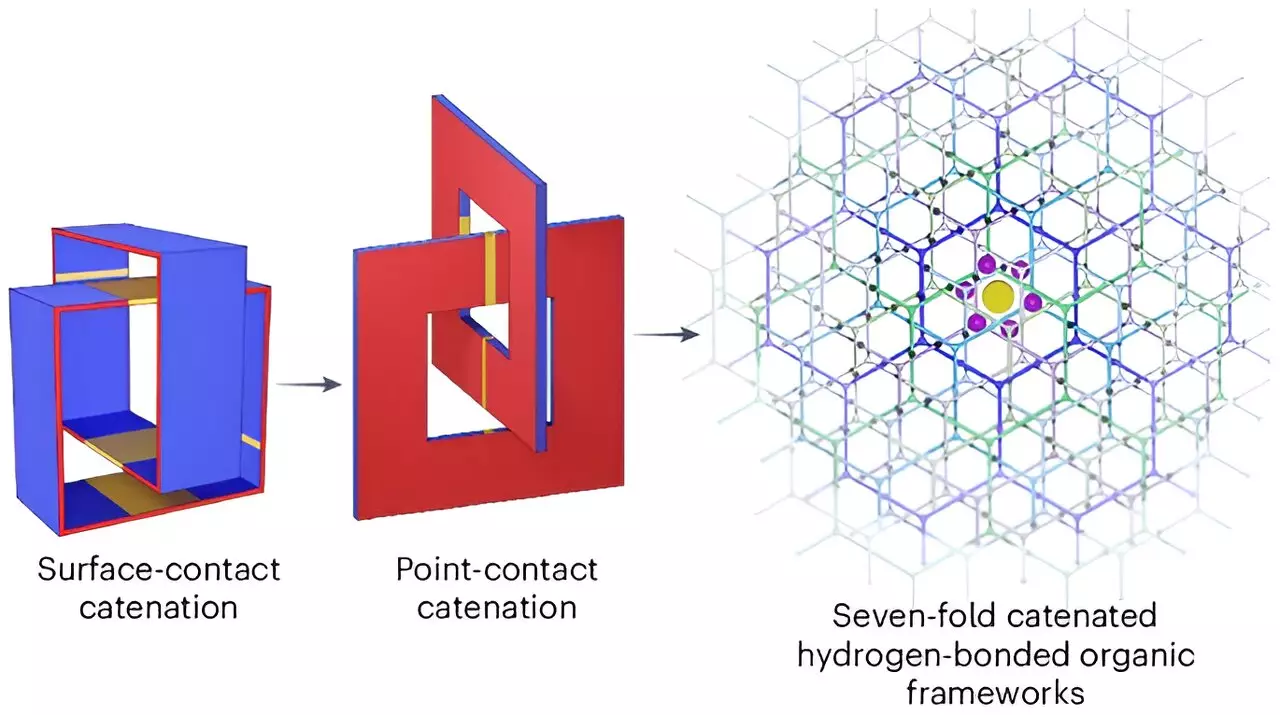
A groundbreaking study carried out by a collaborative team from the University of Hong Kong, Northwestern University, and Duke University presents a promising advancement in hydrogen storage technology. Their findings, published in the esteemed journal *Nature Chemistry*, reveal the development of a lightweight supramolecular material capable of compressing hydrogen efficiently. The new approach employs porous organic crystals designed in a carefully structured honeycomb arrangement. This configuration not only maximizes the intermolecular space for hydrogen storage but also maintains structural integrity and stability, vital for practical applications.
The researchers’ innovative material manages to meet ambitious targets set by the U.S. Department of Energy, which stipulate a hydrogen storage density of at least 50 grams per liter. Moreover, the material must ensure that the weight of the stored hydrogen constitutes at least 6.5% of the total weight of the storage medium. Historically, most attempts to reach these benchmarks have fallen short, adding to the excitement surrounding this new discovery.
The team’s creation is characterized by its array of robust organic molecules interlinked in a manner that favors efficient hydrogen bonding. The pores engineered in the crystalline structure are meticulously sized for optimal hydrogen absorption, effectively securing the gas while minimizing leakage. Testing revealed that the new material could store an impressive 53.7 grams of hydrogen per liter, exceeding the targeted density and achieving a ratio where hydrogen comprises 9.3% of the material’s overall weight.
Despite these promising results, the researchers acknowledged a significant limitation: the necessity of cryogenic cooling for the system to function effectively. This requirement could pose practical challenges in terms of bulkiness and cost, potentially hindering the material’s commercial viability.
The development of this novel supramolecular material represents a significant milestone in addressing the key challenges of hydrogen storage. While substantial hurdles remain, particularly regarding the cooling requirements, the research illustrates the potential of new materials science techniques in propelling sustainable energy solutions forward. As the global community increasingly seeks alternatives to traditional fossil fuels, advancements like these could play a crucial role in paving the way for a cleaner and greener future. Continued research and development in this domain will be vital for refining these materials further, facilitating their transition from laboratory innovations to real-world applications in the hydrogen economy.

Leave a Reply