In the pursuit of efficient energy conversion and storage technologies, the oxygen evolution reaction (OER) plays a pivotal role, especially in the context of water splitting. One of the primary challenges in this area is achieving stability and efficiency in acidic environments, which are often required for dynamic energy applications. The traditional reliance on precious metals has hindered broader adoption due to cost and availability issues. However, recent advancements in electrocatalyst development promise to change the game, particularly with the innovative approach taken by researchers incorporating erbium into cobalt oxide.
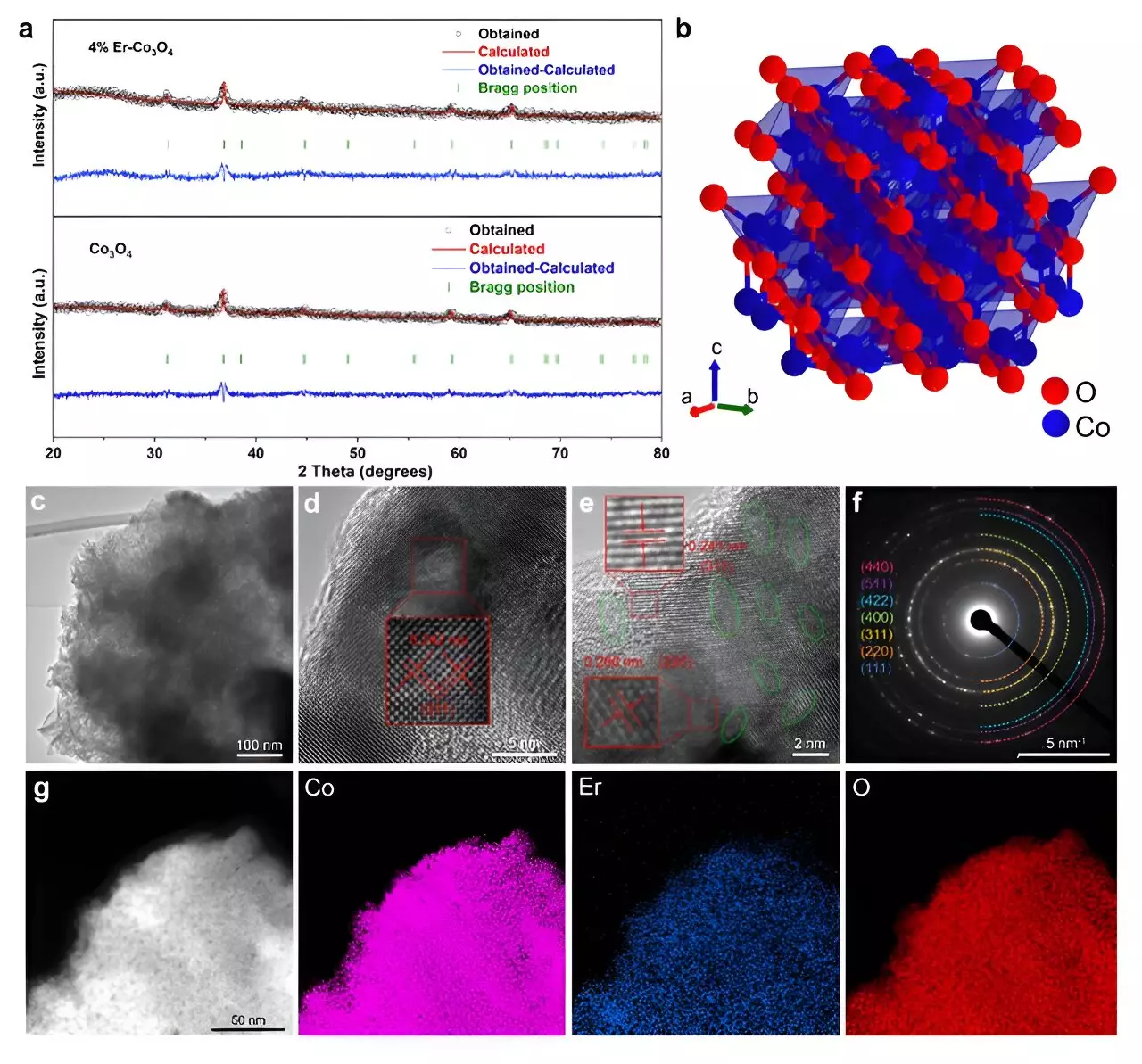
A team of researchers has made a groundbreaking observation by integrating the rare earth element erbium (Er) into cobalt oxide (Co3O4). This combination not only enhances the performance of the catalyst but also provides a cost-effective alternative to conventional noble metal catalysts. The findings of this study, documented in the journal ACS Catalysis, highlight an exciting development in the field of catalysis, one that was even recognized as an Editors’ Choice by the journal.
By doping Co3O4 with a mere 4% Er, the researchers have achieved a remarkable overpotential of just 321 mV at a current density of 10 mA/cm². This performance places the erbium-doped catalyst on par with, and in some instances superior to, traditional iridium-based catalysts, renowned for their high activity but expensive formulation.
To appreciate the significance of this advancement, it is crucial to delve into the underlying mechanisms that contribute to the enhanced performance of the doped catalyst. The researchers employed sophisticated methods, including density functional theory (DFT) calculations and microkinetic modeling. These analyses revealed that the addition of Er results in a transformation within the cobalt oxide’s crystal structure. Specifically, it creates more active sites and defects, thus increasing the ratio of Co3+ to Co2+ ions.
This shift is vital as it generates a greater number of oxygen vacancies, which are instrumental in accelerating the OER process. The analogy presented by Hao Li, an associate professor involved in the study, illustrates this concept well: “Imagine the catalyst like a road… the Er doping essentially adds extra lanes, allowing more traffic to move through smoothly.” This “traffic” refers to the oxygen intermediates necessary for facilitating the reaction, emphasizing how the introduction of erbium optimizes the catalyst’s functionality.
Experimental Verification and Insights
The researchers utilized in situ Raman spectroscopy to gain insight into the structural changes induced by erbium doping. This technique allowed for the identification of oxygen vacancies located within the octahedral sites of the Co3O4 lattice. These vacancies not only increased the formation of essential intermediate species but also bolstered the availability of reactive Co3+ sites, integral for the catalytic activity.
The synergistic effect of electronic reconfiguration and structural modifications illustrates a sophisticated interplay that boosts the overall performance of the catalyst. This balance of factors is pivotal for the advancement of efficient energy conversion technologies.
The implications of this research extend far beyond the laboratory, potentially revolutionizing the field of electrocatalysis. By demonstrating a pathway to develop high-performance, non-precious metal catalysts, the researchers provide a glimpse into a more sustainable future. Their ongoing work aims to explore further non-precious metal dopants, looking to refine and expand the range of accessible, effective catalysts.
As the demand for green energy solutions intensifies, innovations like the erbium-doped cobalt oxide catalyst may play a crucial role in making advanced energy technologies more available and affordable. The prospect of using cost-effective materials to achieve high catalytic performance opens avenues for broader applications in various sectors, heralding an era of sustainable energy practices.
The introduction of erbium into cobalt oxide reflects a significant step toward overcoming the historical reliance on expensive catalysts, suggesting a promising pathway for future research and development in the field of electrocatalysis.

Leave a Reply