The emerging field of high entropy oxides has gained considerable attention from researchers due to their unique structural and functional properties, which play a crucial role in various electronic applications. Recent findings published in the Journal of the American Chemical Society delve into the significant influence that different synthesis methodologies can have on these materials. This article explores these findings, expanding on the implications for materials science and their potential applications.
High entropy oxides are a diverse range of materials characterized by their ability to incorporate multiple transition metal oxides into a singular crystal lattice. This diversity grants them a remarkable level of chemical flexibility, making them particularly attractive for applications in energy storage and conversion, catalysis, and beyond. As noted by material scientists, the ability to manipulate the composition and synthesis conditions of these oxides opens up expansive opportunities for developing materials with tailored properties.
Recent research has focused on high entropy oxides with a spinel crystal structure, exemplifying a blend of various transition metal oxides. This study, led by a team at the University of British Columbia’s Blusson Quantum Matter Institute, specifically investigates how the choice of synthesis method can lead to variations in local and microstructural characteristics of these materials—insights that were previously not well understood.
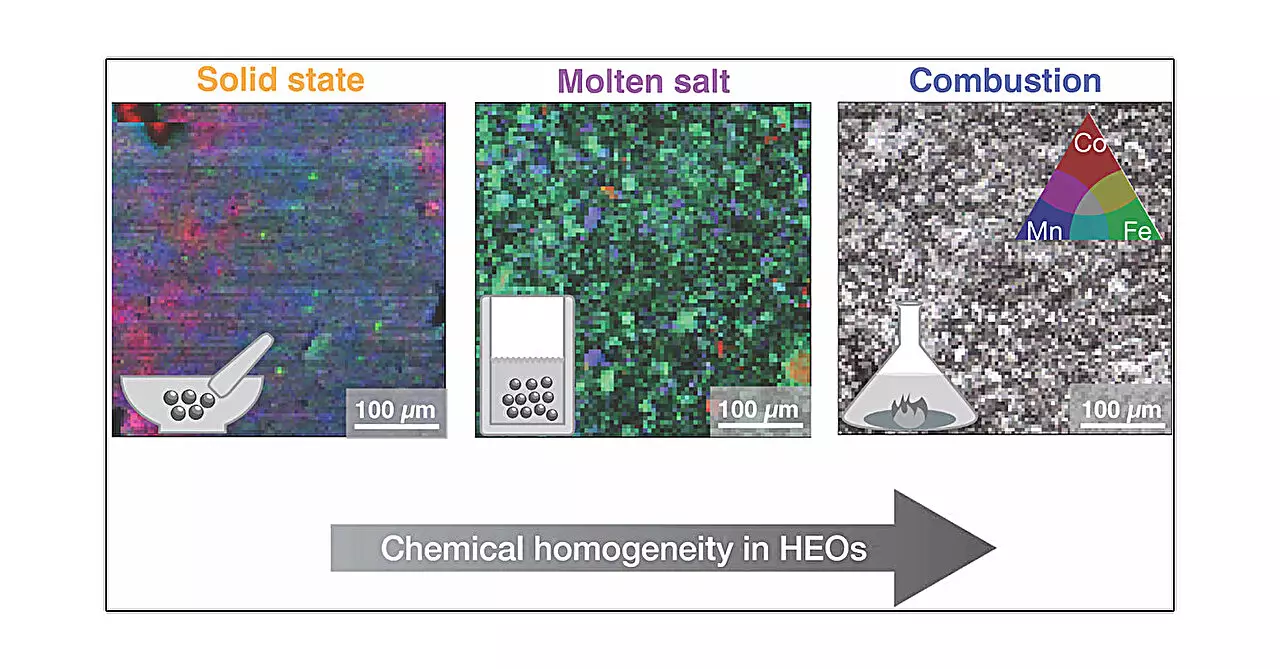
The research drew comparisons between five distinct synthesis techniques: solid state, high pressure, hydrothermal, molten salt, and combustion. Each technique employs different physical and chemical conditions to manipulate how materials crystallize and develop their microstructures. For instance, the solid-state synthesis is akin to traditional baking, where metal oxides are mixed and heated together, while high-pressure synthesis introduces additional force that can alter the final structure. Hydrothermal processes, on the other hand, replicate natural mineral formation conditions by utilizing water within a pressurized vessel.
A particularly noteworthy method is the combustion synthesis, where a gel-like mixture of metal salts ignites to produce high temperatures, yielding materials almost instantaneously. This rapid formation can dramatically affect the integrity and uniformity of the resulting oxides. The study identified combustion synthesis as yielding the most homogeneous structural characteristics, which may be advantageous for applications requiring consistent performance.
One of the most significant outcomes of this research is the emphasis placed on the importance of microstructure. While it was observed that the average properties of the materials remained comparable across different synthesis methods, the local variations in structure and microstructural arrangement were profound. This insight is crucial, as the functional properties of materials often hinge on their microstructural characteristics.
For instance, in applications such as energy systems, the small-scale architecture of the oxide can influence how electrons flow, how ions move, and ultimately how effectively the material performs in practical scenarios. As Mario Ulises González-Rivas, the lead author of the study, articulates, these important variations provide significant avenues for optimization in applied settings.
Implications for Future Research and Application
The findings of this study encourage a reconsideration of how materials scientists approach the synthesis of high entropy oxides. Instead of solely focusing on achieving uniform chemical compositions and high purity, future research could benefit greatly from understanding and controlling microstructural variations brought about by different synthesis techniques.
Furthermore, the collaboration between institutions, such as the University of British Columbia, the University of Saskatchewan, and the Max Planck Institute, illustrates a growing trend toward interdisciplinary approaches in material science. The combination of varying expertise can foster innovations that may lead to superior materials with enhanced properties.
This new research unveils the complex interplay between synthesis methods and material characteristics in high entropy oxides. By recognizing and manipulating synthesis variables, researchers can pave the way for advancements in energy technologies, catalysis, and other high-performance applications, highlighting the ongoing journey into the potential of high entropy materials.
Leave a Reply