For decades, the focus of biological chemistry has predominantly rested on the well-established elements of cellular function: protein folding, gene activity, and electrical signaling pathways. These components have long been regarded as critical elements responsible for maintaining homeostasis and the overall health of living organisms. Recent studies, however, have revealed a novel class of structures known as biological condensates that are challenging the traditional understanding of cellular mechanisms. Unlike the proteins and genes that have historically drawn the spotlight, these condensates operate on principles of density and phase separation, akin to oil droplets suspended in water. This groundbreaking perspective suggests that these cellular “blobs” may hold the keys to new therapeutic strategies and unconventional regulatory mechanisms in cellular biochemistry.
The Nature and Function of Biological Condensates
Biological condensates are fascinating structures that arise in cells as a result of differential density without the need for rigid membranes. Their ability to form dynamic compartments allows them to sequester and manipulate a variety of biomolecules—proteins, enzymes, ions, and more—playing a decisive role in cellular operations. Studies have indicated that these structures can either concentrate certain molecules to enhance their activity or isolate them to reduce their functionality. This selective partitioning hints at a nuanced level of biochemical regulation that had previously received little attention.
Further exploration has revealed that biological condensates can create unique electrochemical environments within cells. By accumulating positively or negatively charged ions, these condensates can influence adjacent cellular membranes, modifying the electrostatic landscape critical for multiple cellular processes. This suggests that condensates are not merely passive aggregations of molecules but active participants in cellular signaling and energy regulation.
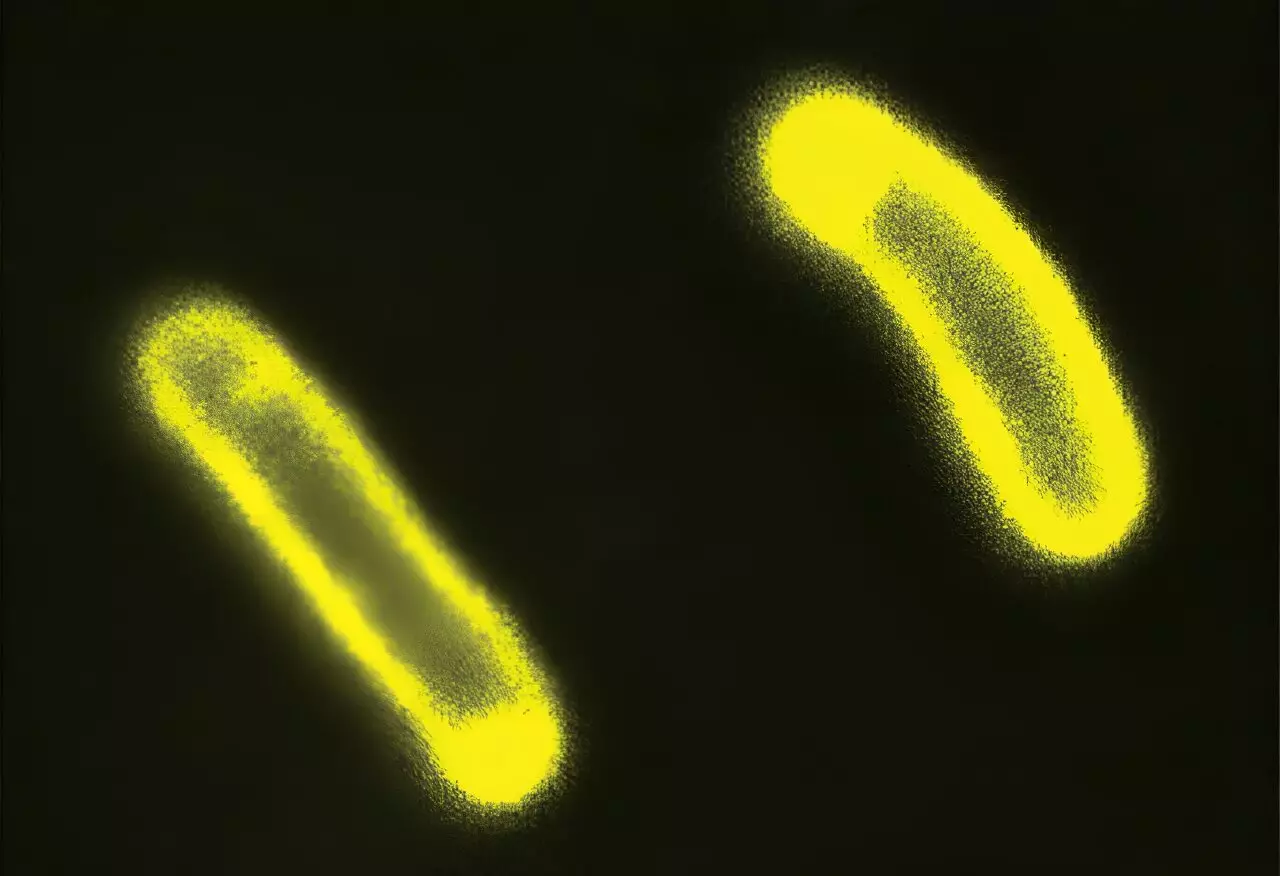
A significant advancement in our understanding of biological condensates emerged from a recent study published in the journal Cell, involving researchers from Duke University and Washington University in St. Louis. This work illuminated a previously unexamined mechanism by which biological condensates influence cellular activity over greater distances—not just within their immediate vicinity. As Professor Lingchong You noted, the findings suggest a “wireless connection” by which condensates modulate interactions between cells and their environments. This perspective on cellular communication could reframe our understanding of how cells adapt to various stresses, particularly in the context of antibiotic resistance.
The study demonstrated that condensate formation could alter the electrical properties of cellular membranes, leading to changes in the cell’s susceptibility to antibiotics. The researchers meticulously engineered conditions under which E. coli bacteria formed internal condensates and subsequently measured alterations in the charges of their cellular membranes. Their results revealed a direct correlation between the presence of condensates and variations in cellular response to antibiotics, highlighting a potentially transformative discovery in the field of microbiology and pharmaceutical development.
The Broader Implications of Condensate Research
While the study’s findings sparked excitement, it is critical to acknowledge that we might only be scratching the surface of understanding cellular condensates. As highlighted by Professor Ashutosh Chilkoti, the implications of this research extend far beyond antibiotic resistance, suggesting that condensates could play a role in diverse cellular behaviors and adaptations. With growing evidence that condensates impact gene regulation on a global scale, there is a compelling case for further investigation into how these structures might influence various diseases, cellular differentiation, and responsiveness to therapies.
Moreover, the potential applications of leveraging biological condensates in therapeutic contexts are vast. If researchers can identify ways to harness or manipulate these structures, they may pave the way for novel treatments for diseases linked to cellular misregulation, including cancers and neurodegenerative disorders.
The investigation into biological condensates is an exciting avenue in biochemistry that warrants serious attention. As more studies emerge, we may witness a paradigm shift in our comprehension of cellular dynamics, revealing complex interdependencies and mechanisms of action that have eluded scientists until now. The implication that these unassuming cellular structures can orchestrate significant physiological changes underscores a vital need for continued exploration. The journey into the world of biological condensates could reshape not only our understanding of cell biology but also our approaches to treating diseases, presenting an enlightening challenge to entrenched paradigms in the life sciences.

Leave a Reply