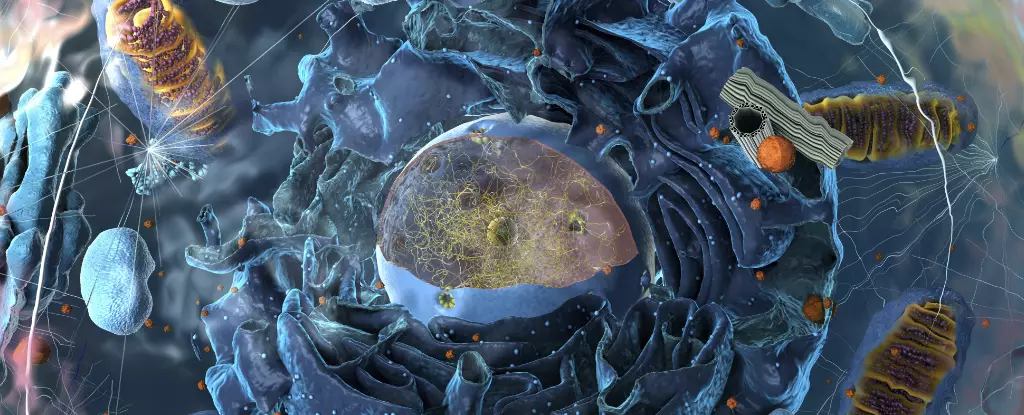
Biological education often revisits the foundations established in earlier academic settings, but recent discoveries may reshape these foundations fundamentally. Traditional cell biology has long been anchored in the notion that every organelle within a cell is encased in a membrane, creating distinct compartments with unique functions. This understanding has been transformed through the advent of research focused on biomolecular condensates—organelles devoid of membranes that have unveiled critical insights into cellular chemistry and the origins of life itself.
For years, the scientific community was anchored to the idea that membrane-bound organelles like mitochondria, lysosomes, and the nucleus defined cellular functionality and organization. However, the mid-2000s unveiled a paradigm shift: researchers uncovered various organelles that function effectively without a protective membrane. These newly identified structures, known as biomolecular condensates, challenge traditional definitions and have led to a proliferation of inquiry into their functions and implications.
Biomolecular condensates can be visualized as dynamic entities resembling lava lamps, where clusters of proteins and RNA intermingle in a gel-like state. This distinct behavior arises from their specific interactions; certain proteins and RNA molecules preferentially engage with each other over the surrounding cellular environment, creating specialized biochemical compartments. As of 2022, scientists have identified around 30 unique types of these condensates, vastly outnumbering the traditional membrane-bound organelles. The recognition of these condensates has prompted a radical reevaluation of cellular mechanics and their potential roles.
Despite the increasing catalog of identified biomolecular condensates, understanding their specific functions remains a complex challenge. Some condensates perform well-established roles such as facilitating protein synthesis or forming stress granules, while others elude definitive characterization. This ambiguity highlights a fundamental aspect: the functional diversity of membraneless organelles may far exceed what was previously recognized with traditional organelles. Understanding the nuances of these condensates will undoubtedly alter the scientific community’s fundamental understanding of cellular operations.
A significant departure from earlier beliefs arises from the nature of the proteins involved in the formation of biomolecular condensates. Traditionally, a clear structure was believed to dictate a protein’s function. However, many proteins in biomolecular condensates exhibit regions known as intrinsically disordered proteins (IDPs), which lack the stable structures typically associated with functionality. This realization challenges the long-standing notion equating protein shape with functionality, now prompting biochemists to reconsider the nature of functional proteins in the cell.
The implications of biomolecular condensates extend beyond eukaryotic cells; they also appear in prokaryotic organisms, traditionally defined as lacking organelles. This revelation has significantly transformed our understanding of bacterial biology. While it was estimated that only a minority of bacterial proteins possess disordered regions, the presence of biomolecular condensates in these microorganisms suggests a level of complexity previously unacknowledged. These condensates may play integral roles in essential cellular processes, including RNA synthesis and degradation.
This newfound complexity compels a rethinking of what constitutes a prokaryotic cell. Bacteria can no longer be simplistically labeled as mere sacks of biomolecules; their organization and cellular function are far more intricate and nuanced than conventional interpretations suggest.
As research into biomolecular condensates unfolds, it prompts significant reflections on the origins of life on Earth. Current hypotheses posit that basic cellular components, like nucleotides, could arise from simple chemical reactions under specific conditions. However, traditional views often assert that nucleic acids, particularly RNA, must encapsulate within lipid-based membranes to form proto-cellular structures, a proposition that hinges on the availability of lipids in early Earth conditions.
The emergence of evidence detailing how RNA molecules could independently aggregate into biomolecular condensates challenges this paradigm. If RNA transitions to functional aggregates without lipid membranes, then the door opens to the possibility that the inception of life could be attributed to simple organic chemistry rather than complex lipid synthesis.
For researchers engrossed in the study of biomolecular condensates, excitement thrives in the prospect of revolutionizing biological understanding. These entities are poised not only to alter foundational teachings but also have the potential to influence medical research, especially in understanding diseases characterized by protein aggregation, such as Alzheimer’s and other neurodegenerative disorders. As new methodologies develop to manipulate these condensates for therapeutic use, the implications could be transformative.
The revelation of biomolecular condensates stands as a testament to the ever-evolving nature of biology. Far from traditional education’s static portrayal, the field is rife with discovery and reevaluation, suggesting a bright future where each biomolecular condensate may earn its rightful place in the annals of scientific knowledge. From high school classrooms to advanced research labs, the story of cellular biology is being rewritten, beckoning an era of deeper understanding and exploration.
Leave a Reply