Hydrogen, the lightest and most abundant element in the universe, has emerged as a critical player in the quest for sustainable energy. Its isotopes—protium, deuterium, and tritium—offer unique properties and applications in various sectors, from pharmaceuticals to nuclear fusion. However, the separation of these isotopes has historically been fraught with challenges due to their similar physical characteristics. Recent advancements by a collaborative team from Leipzig University and TU Dresden, particularly under the Hydrogen Isotopes 1,2,3H Research Training Group, have sparked excitement in the scientific community. Their breakthrough in separating hydrogen isotopes at ambient temperatures presents a viable pathway towards an efficient, cost-effective provision of these vital resources.
To anchor our discussion, we must recognize the three isotopes of hydrogen. Protium (hydrogen-1) is the most prevalent isotope and found in vast quantities across the globe. Deuterium (heavy hydrogen) has gained traction in recent years due to its potential in producing more stable pharmaceuticals. Tritium, coupled with deuterium, forms “super-heavy” hydrogen, a critical component in the ongoing search for sustainable nuclear fusion energy. The immense promise that these isotopes hold renders their efficient and pure extraction paramount.
However, the inherent similarities among the isotopes complicate their separation. Current methodologies tend to be energetically expensive and inefficient, burdening the industries that rely on them. Therefore, the quest for effective separation techniques remains a crucial pursuit in advancing hydrogen research.
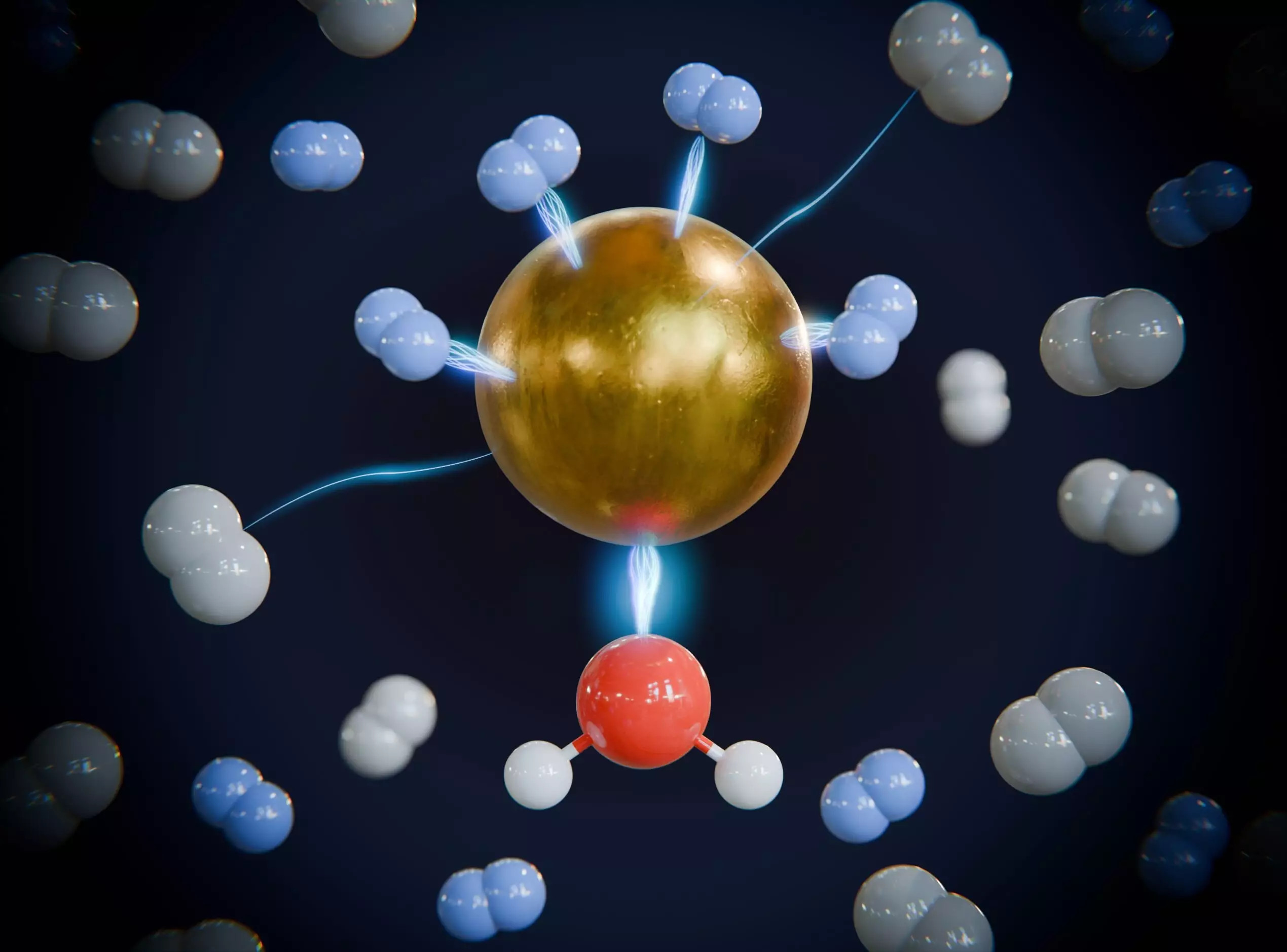
For nearly 15 years, there has been speculation regarding the potential of porous metal-organic frameworks (MOFs) in isolating hydrogen isotopes. Historically, processes utilizing these frameworks have required ultralow temperatures, around -200 degrees Celsius, making them impractical for large-scale applications. The recent research by the Leipzig-Dresden collaboration, however, suggests that separation could occur at room temperature, opening doors to industrial feasibility and cost reduction.
Professor Knut Asmis, along with a team of dedicated doctoral researchers, has diligently investigated the underlying principles that dictate the adsorption behaviors of these isotopes within the framework materials. Adsorption, wherein atoms or molecules adhere to a solid surface, plays a pivotal role in the efficacy of the separation process. Through advanced spectroscopy techniques and quantum chemical calculations, the research team has discerned how the specific atomic makeup of framework compounds influences isotope binding.
The implications of these findings cannot be overstated. By elucidating the factors that affect isotope binding selectivity, the research teams are now positioned to tailor materials to optimize their effectiveness in separating hydrogen isotopes. Such advancements could fundamentally alter how these isotopes are sourced and utilized, enhancing their accessibility for diverse applications such as energy production and pharmaceutical development.
Moreover, this work underscores the synergy between theoretical calculations and experimental methodologies. By combining insights from spectroscopy with quantum chemistry, the researchers have not only tackled a longstanding issue but have also paved the way for a more integrated approach in future hydrogen research.
As the race towards sustainable energy intensifies, the role of hydrogen continues to grow. The accomplishments of the Leipzig and Dresden research teams signify a major leap forward in our ability to harness hydrogen’s potential. The development of cost-effective methods to separate hydrogen isotopes at room temperature could catalyze advancements across multiple fields, especially as nations seek cleaner energy alternatives.
While challenges remain in the hydrogen research landscape, innovative studies such as those conducted by the Hydrogen Isotopes 1,2,3H Research Training Group illuminate the path forward. The future of energy may very well hinge on our ability to unlock the secrets of hydrogen isotopes effectively, and recent developments suggest we are on the right track.
Leave a Reply