Samarium (Sm) is a rare earth metal gaining traction among organic chemists due to its unique properties, particularly its divalent compounds, which excel in facilitating single-electron transfer reductions. Among its various derivatives, samarium iodide (SmI2) stands out for its moderate stability and efficacy under relatively mild, ambient conditions. These characteristics make it an essential reagent in the synthesis of pharmaceuticals and biologically active compounds. However, this utility is accompanied by significant limitations, primarily concerning the need for stoichiometric amounts of Sm in most reactions, which often involve hazardous chemicals, escalating safety concerns and costs.
The journey towards more sustainable and cost-effective applications of Sm has been fraught with difficulty. The conventional methods often require harsh conditions and substantial quantities of the metal, typically around 10% to 20% of the total raw materials. This reliance not only burdens the environment due to waste generation but also increases the financial implications associated with obtaining such an expensive rare earth element. As a result, the demand for innovative catalytic systems that minimize the use of Sm while maintaining efficiency and effectiveness has become increasingly pronounced.
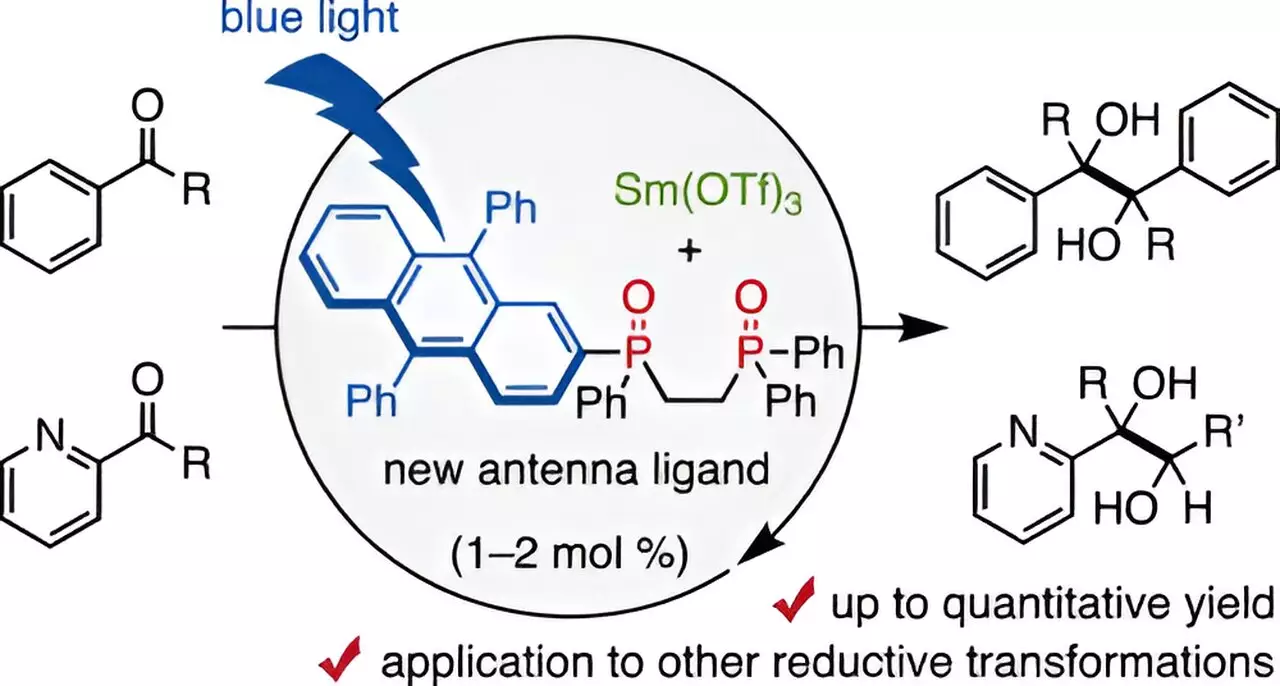
Recently, researchers from Chiba University in Japan, led by Assistant Professor Takahito Kuribara, have made compelling strides toward addressing these issues. Their investigation centered around the development of a novel bidentate phosphine oxide ligand, specifically designed to coordinate with trivalent samarium and harness the power of visible light for catalysis. This new ligand, which they aptly named a “visible-light antenna,” demonstrates the potential to dramatically reduce the necessary amount of the Sm catalyst.
Kuribara’s team comprises prominent researchers, including Ayahito Kaneki, Yu Matsuda, and Tetsuhiro Nemoto. Their findings, published in the Journal of the American Chemical Society, highlight a groundbreaking shift in the way Sm can be employed in organic reactions.
Through a carefully designed series of experiments, the team established that by combining the Sm catalyst with their designed DPA-1 ligand under blue-light irradiation, they could produce remarkable yields of up to 98% in pinacol coupling reactions involving aldehydes and ketones—two classes of compounds frequently utilized in pharmaceutical syntheses. Crucially, they discovered that only 1% to 2% of Sm was needed for these reactions, a substantial reduction from previously required stoichiometric levels.
Interestingly, the presence of mild organic reducing agents, such as amines, further distinguished this approach from traditional methods, which typically call for highly reactive substances. The optimal conditions identified also indicated that while a small quantity of water improved yields, excessive water could hinder the reaction process, underscoring the need for precise control in reaction environments.
To delve deeper into the efficacy of the DPA-1 ligand, the researchers analyzed its photophysical properties when combined with the Sm catalyst. They learned that DPA-1 excels not just in coordinating with Sm but in selectively absorbing blue light and transferring electrons efficiently from the ligand to the metal. This crucial function serves as a pivotal mechanism facilitating the observed high efficiency and catalytic activity.
The versatility of the Sm catalyst coupled with DPA-1 extends beyond simple reactions; it has been successfully applied to a range of molecular transformations essential in drug development. This approach integrates Sm-based reduction and photo-oxidation, further highlighting the innovation presented by Kuribara’s team.
The advancements achieved by the team at Chiba University represent a transformative leap in the field of organic chemistry. By dramatically reducing the cathodic requirements of Sm from stoichiometric to catalytic levels, they have not only alleviated the burden associated with resource use but also opened doors to more sustainable and environmentally friendly synthetic processes. The development of visible-light antenna ligands stands as a testament to the potential of innovative ligand design in catalysis, signaling a promising future for Sm-based reactions.
As further research unfolds, this work paves the way for the continual evolution of efficient catalytic systems, bridging the gap between high-performance organic synthesis and the pressing need for sustainability—an essential endeavor in today’s chemical landscape.

Leave a Reply