Ovarian cancer remains one of the deadliest cancers affecting women worldwide, particularly with the prevalence of high-grade serous ovarian carcinoma (HGSOC), the most aggressive and common type. Recent research focusing on murine models has illuminated potential pathways for early detection and intervention, specifically by pinpointing the cells responsible for the initiation of this cancer. The study, conducted by a team led by Alexander Nikitin at Cornell University, presents vital insights that could transform how this malignancy is diagnosed and treated in humans.
Traditionally, many believed that ovarian tumors developed primarily within the ovaries themselves. However, growing evidence has shifted this perspective toward the fallopian tubes as the initial site for many cases of ovarian cancer. Researchers have noted the presence of lesions and specific genetic markers in the fallopian tubes that are inherently linked to the eventual development of ovarian tumors. Despite these advancements, a significant gap remains in identifying the exact cellular origins of HGSOC within the fallopian tubes. This gap hinders the development of diagnostic markers crucial for early detection.
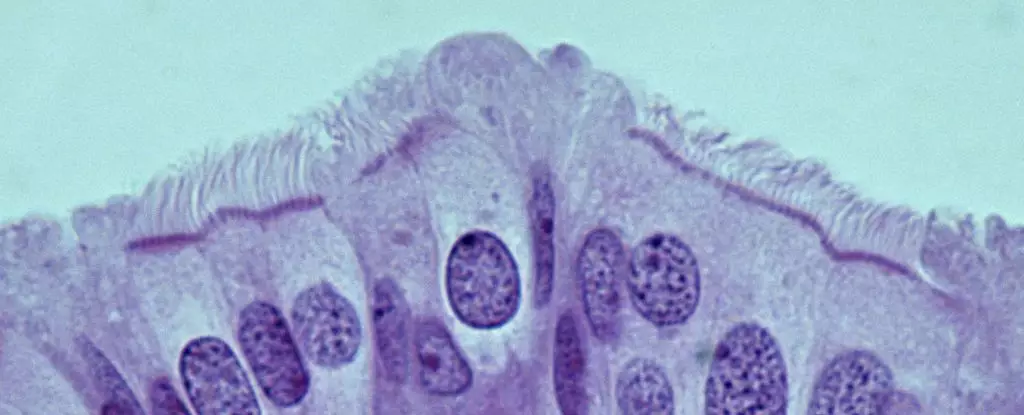
Nikitin’s group, through their animal model, has taken a critical step by isolating and characterizing the various cell types found in the oviducts of mice—the rodent equivalent of human fallopian tubes. Their findings emphasize a paradigm shift: cancer risk within these structures does not reside with stem cells, as previously hypothesized, but rather within pre-ciliated cells undergoing transformation. Understanding the significance of these transitional cells highlights the complexity of cellular behavior in cancer genesis.
The intricacies of how cancer forms in the pre-ciliated cells are particularly compelling. The research reveals that these cells, which play a crucial role in routing oocytes through the reproductive tract, display abnormal behavior when subjected to genetic mutations linked with HGSOC. Specifically, two well-known mutations—a significant focus for cancer genetics—interfere with the normal development of these pre-ciliated cells, thereby promoting an environment conducive to cancer formation.
Moreover, the implications of the study extend beyond ovarian cancer alone; issues related to the formation of cilia in these cells may also connect to other cancers, such as pancreatic cancer. This intersection of research underscores a broader biological principle that highlights how seemingly distinct cancer types can share common mechanistic pathways, thus providing an avenue for more extensive research and therapeutic strategies.
While these findings are promising, they also open a floodgate of questions. Can we establish a definitive link between the identified cellular pathways in mice and their human counterparts? Will this newfound knowledge lead to specific biomarkers that can be utilized for earlier diagnosis? Nikitin and his team acknowledge that substantial work remains. They stress the importance of further exploring the mechanisms by which HGSOC arises in humans, along with examining other genetic mutations that play a role in HGSOC development.
This groundwork is essential not only for enhancing detection methodologies but also for formulating preventive strategies that can ultimately improve patient outcomes. The identification of pre-ciliated cells as a focal point in ovarian cancer genesis could pave the way for innovative therapeutic approaches aimed at targeting these critical cells before they can evolve into full-blown malignancies.
The path forward in combating ovarian cancer hinges on our evolving understanding of its origins. With sustained research efforts, scientists might unlock the potential for earlier detection and more effective treatments, saving countless lives affected by this formidable disease. The confluence of microscopic cellular analysis with genetic insights marks a hopeful turning point in the fight against one of women’s leading health threats.

Leave a Reply