In the quest for sustainable chemical synthesis, chemists have sought alternatives to traditional catalyst systems, particularly those reliant on biological proteins. Researchers at the National University of Singapore (NUS) have emerged as pioneers in this domain, introducing a groundbreaking method that merges DNA repair mechanisms with biorthogonal chemistry to create a versatile platform for producing chiral deoxyribonucleic acid (DNA) catalysts. This development not only addresses some of the inherent challenges of enzyme catalysis—such as instability and complex manipulation of proteins—but also paves the way for a new era of asymmetric catalysis that could transform how chemists approach chiral molecule synthesis.
The utilization of DNA as a scaffold for catalysis represents a significant departure from conventional methods. Proteins, while effective for catalyzing reactions, often come with limitations including the need for intricate design and susceptibility to degradation. In contrast, DNA offers a more stable and cost-efficient option. The inherent programmability of DNA, stemming from its unique base-pairing system, enables precise structural control and functional versatility. This aspect makes DNA an appealing alternative for chemists engaged in asymmetric reactions, as it allows for a broad range of functionalities without compromising the overall performance of the catalyst.
Simplifying DNA Catalysis
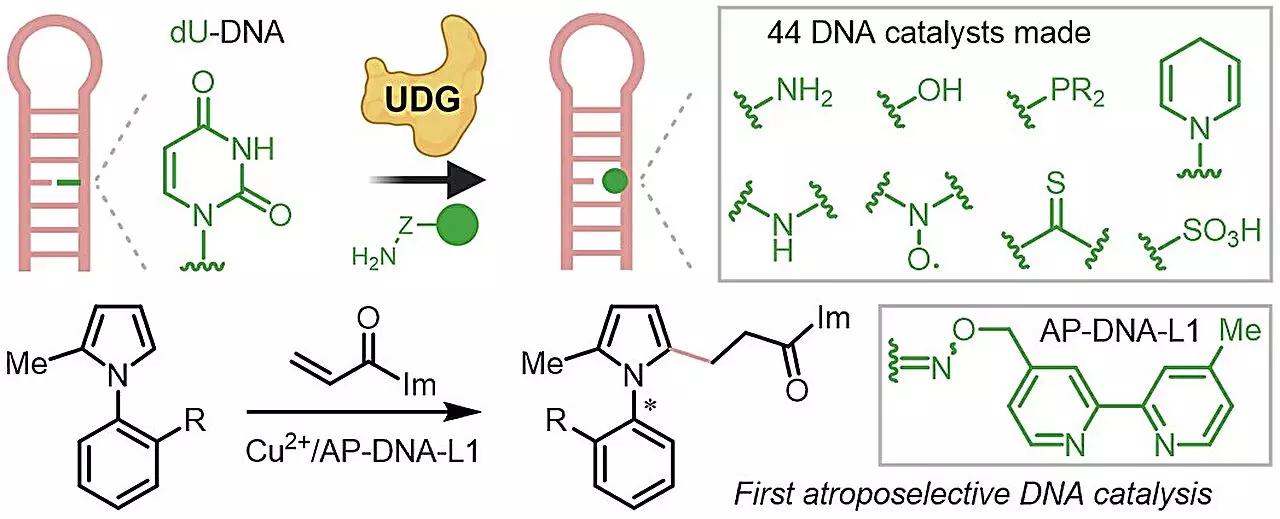
Under the guidance of Assistant Professor Zhu Ru-Yi, the NUS research team has developed a methodology that streamlines the production of chiral DNA catalysts. By leveraging the DNA repair process alongside biorthogonal chemistry, the researchers have made it possible for even those without specialized training to engage in DNA-catalyzed reactions. This democratization of the technique opens the door for greater participation in the field of synthetic chemistry, enabling researchers from diverse backgrounds to utilize these methods effectively.
The NUS team’s findings, published in the esteemed Journal of the American Chemical Society, reveal a remarkable advancement in the functionality of DNA catalysts. By compiling a library of 44 distinct catalysts, they demonstrated that their novel approach not only improved enantioselectivity—a crucial parameter for creating chiral molecules—but also expanded the substrate range and elevated the overall efficiency of reactions. More impressively, the researchers achieved the first known instance of atroposelective DNA catalysis—successfully generating axial chiral compounds, which are notoriously difficult to synthesize using traditional bio-catalysis techniques.
The implications of this research extend far beyond the immediate findings. The robustness of the developed method, showcased by its ability to generate various DNA catalysts with unprotected functional groups, heralds a new frontier in sustainable chemical reactions. Looking forward, the NUS team is poised to explore additional strategies that leverage DNA catalysis for selective and efficient chemical transformations. This innovative approach represents a promising step toward more sustainable practices in both academic and industrial chemistry, emphasizing the importance of interdisciplinary collaboration in tackling some of the most pressing challenges in synthesis today. With ongoing developments in this area, DNA could play a pivotal role in advancing the future of asymmetric catalysis.

Leave a Reply