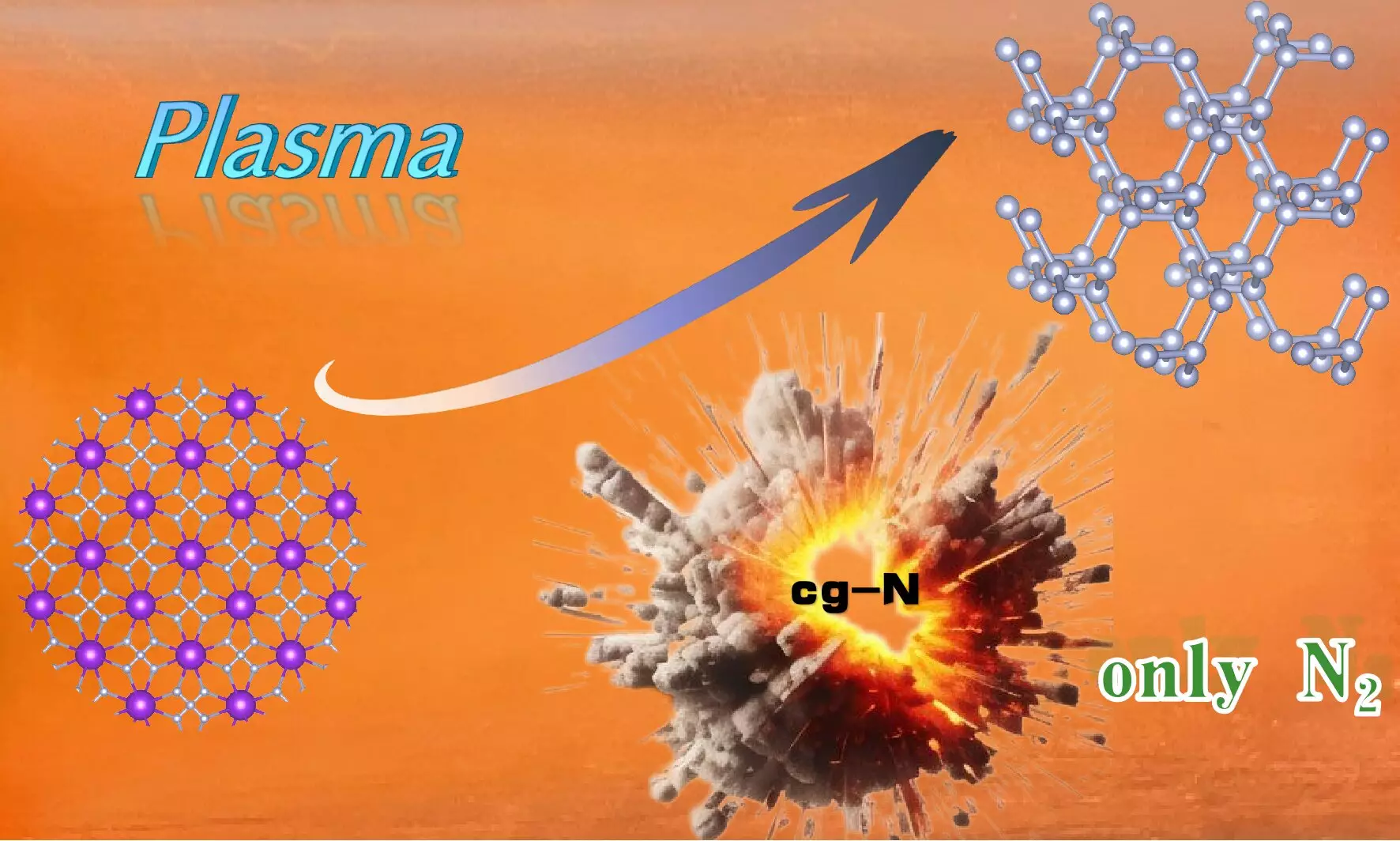
Recent advancements in material science have led to the synthesis of cubic gauche nitrogen (cg-N), a high-energy-density compound with unique structural properties. The research team led by Prof. Wang Xianlong, associated with the Hefei Institutes of Physical Science under the Chinese Academy of Sciences, has made a significant breakthrough by developing a method to synthesize this nitrogenous material at atmospheric pressure. This innovative approach has garnered attention within the scientific community, as it opens avenues for safer and more efficient production processes for high-energy-density materials.
Cubic gauche nitrogen is strikingly different from traditional nitrogen compounds due to its diamond-like structure, characterized by nitrogen atoms connected by N-N single bonds. This configuration not only enhances its energy density but also ensures that it decomposes exclusively into nitrogen gas. This characteristic is particularly desirable for applications in propulsion and energy storage, where safety and environmental considerations are paramount. The achievement of synthesizing cg-N without the complications associated with previous methodologies underscores the potential of this material in future applications.
The research team utilized a novel technique known as plasma-enhanced chemical vapor deposition (PECVD) to synthesize cg-N. This method involved treating potassium azide (KN3), chosen for its lower toxicity and explosive nature compared to other precursors. The choice of KN3 was strategic, leveraging potassium’s strong electron transfer capabilities which are crucial for maintaining stability in the cg-N structure. The team effectively demonstrated how manipulating the synthesis environment could result in stable high-energy materials, without relying on more complicated carbon nanotube-limiting effects that have hampered previous research efforts.
One of the unique aspects of this research was the application of first-principles calculations to simulate cg-N’s surface stability. By analyzing various saturated states, pressures, and temperatures, the researchers discovered that the instability at low pressures could lead to cg-N’s decomposition. These insights were pivotal; they guided the team in developing strategies to stabilize cg-N through the modification of surface suspension bonds and the manipulation of charge transfer. Such innovations were essential to synthesize cg-N successfully at atmospheric pressure, maintaining its integrity at elevated temperatures.
Thermogravimetric-differential scanning calorimetry (TG-DSC) tests validated the thermal stability of synthesized cg-N, confirming its resilience up to 760 K before rapid thermal decomposition occurs. These findings serve not only to affirm the material’s stability but also as a stepping stone for future research into high-energy-density materials. The study presents a comprehensive framework for synthesizing cg-N, offering fresh perspectives on how advanced materials can be developed.
The research conducted by Prof. Wang’s team signifies a remarkable step forward in material science. It paves the way for future innovations in the synthesis of high-energy-density materials that could have transformative impacts across various fields, including energy and propulsion technologies. The implications of this work extend beyond immediate applications, potentially leading to further exploration and discovery within the scientific domain.

Leave a Reply