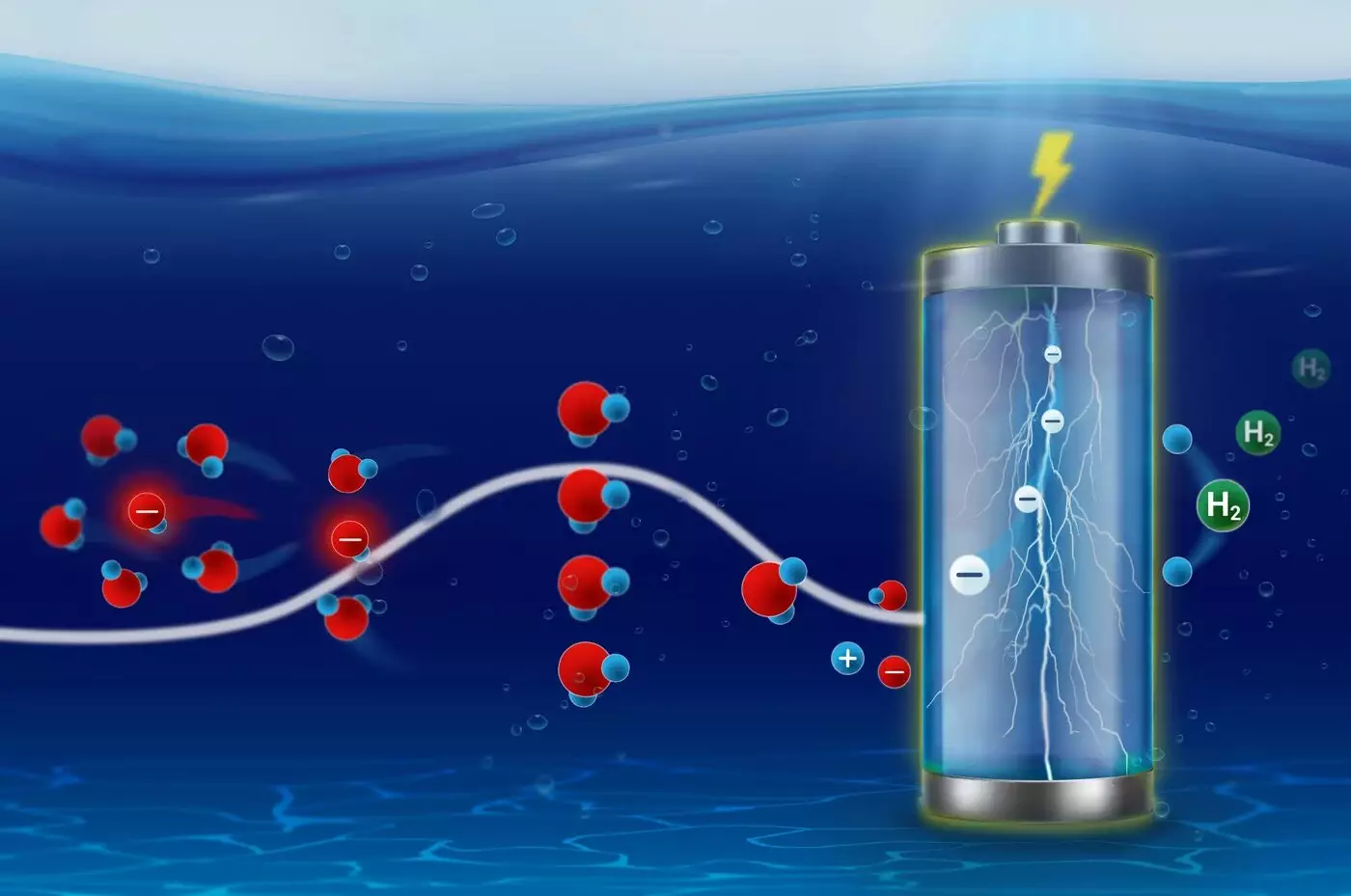
The behavior of ions plays a pivotal role in numerous electrochemical processes, particularly in energy storage and conversion technologies. From the intercalation of lithium ions in battery cathodes to the navigation across ion channels in biological membranes, these charged particles must undergo a fundamental transformation known as solvation. Solvation refers to the process whereby solvent molecules surround and interact with ions, which is crucial before the ions can effectively participate in reactions. The recent investigations by the Interface Science Department at the Fritz-Haber Institute have unveiled the complexities of how solvation kinetics not only influence chemical activity but also provide new insights into electrocatalyze behavior.
In their groundbreaking research published in *Nature Communications*, scientists employed transition state theory, refined by early physical chemists like Michael Polanyi, to elucidate the interfacial reactions of ions. The research hinges on understanding compensation effects between activation entropy and activation enthalpy, which collectively determine the feasibility of ionic mobilization. As the energy barrier for ion movement increases—symbolized metaphorically as a mountain—so too does the number of pathways available for traversal. This relationship is crucial in explaining the kinetics involved in electrocatalytic processes.
By employing millisecond resolution techniques, the researchers successfully measured these activation parameters, enabling them to explore ionic behavior under various conditions. Dr. Sebastian Öner, a key figure in this work, emphasizes the comprehensive nature of their findings, which challenge previously accepted notions regarding activation energies as the primary determinants of electrocatalytic efficiency.
A significant aspect of the findings relates to how pH levels can induce changes in activation entropy, leading to observable deviations in electrocatalytic processes from standard Nernstian behavior. This revelation is pivotal as it suggests that the local environmental conditions exert profound control over the dynamics of chemical reactions occurring at catalyzed interfaces. Understanding these factors can lead to enhanced design principles for catalysts, potentially improving their efficiency and longevity.
The distinction between the effects of activation energy and activation entropy is not merely academic; it has practical implications for the design of catalytic systems in real-world applications, particularly in energy conversion processes such as fuel cells or hydrogen production.
The study also highlights the dynamic interactions occurring at catalyst surfaces during reactions, particularly the phenomenon known as dynamic poisoning. For example, while investigating the ammonia oxidation reaction, researchers found that the platinum surface experiences self-induced substrate changes, which in turn affect the solvation kinetics of hydroxide ions. This ongoing interaction between solid structures and their liquid electrolytes indicates a complexity that must be understood for optimized catalyst design.
Such insights are crucial in advancing the fields of energy storage and conversion technologies, where improving the activity and selectivity of materials is of paramount importance. By uncovering previously hidden aspects of solvation dynamics, researchers can now develop more effective strategies for crafting catalysts that are not only more reactive but also more resilient under varying operational conditions.
The Interface Science Department, under the leadership of Prof. Dr. Beatriz Roldán Cuenya, is set to continue its exploration of these complex systems. The findings underscore the necessity of a unified approach that combines kinetic understanding with spectroscopic and microscopic techniques to fully elucidate the underpinnings of catalytic activity. By establishing a more refined relationship between the solid and liquid phases, there is a pathway toward achieving breakthroughs in energy and chemical conversion technologies.
The study of ion solvation and its kinetics not only deepens our understanding of fundamental electrochemical processes but also opens avenues for innovative solutions in catalyst design. The intersection of theoretical frameworks and real-time monitoring emerges as a vital resource for advancing the field, as researchers strive to meet the global challenges posed by energy sustainability and chemical efficiency. These revelations represent a significant leap forward in both knowledge and application, heralding a new era of exploration in electrochemistry.
Leave a Reply