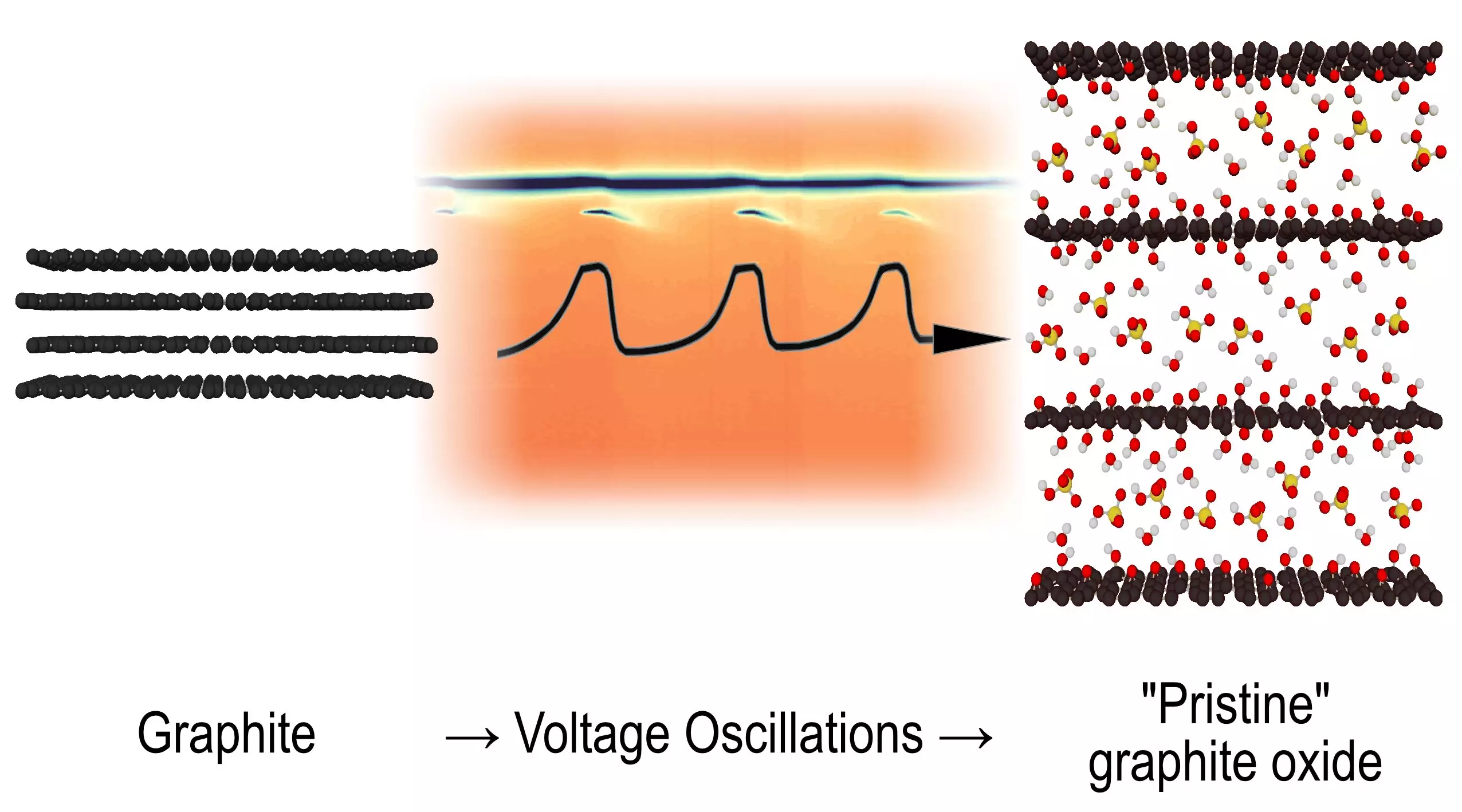
A significant breakthrough in the field of physical chemistry has emerged from Umeå University, where researchers have shed light on a chemical reaction that has puzzled scientists for an astonishing half-century. This study, which was published in the esteemed journal Angewandte Chemie, delves into the transformation of graphite into graphite oxide during electrochemical oxidation. By capturing rapid structural snapshots of the reaction, researchers have uncovered intermediate structures that exhibit a fascinating oscillating behavior. This revelation not only enhances our understanding of chemical reactions but also opens the door to the complexities of chemical systems, drawing a parallel to natural phenomena.
Chemical oscillations, characterized by periodic changes in oxidation states and the appearance of different phases, offer a unique glimpse into the underlying mechanisms of reactions. Traditional illustrations of such reactions often depict visible color shifts in a solution, creating a mesmerizing display of cycling states. In this context, the investigation into graphite oxide formation has offered a deeper insight into the oscillation process. The study reveals that oscillation phenomena were already known, particularly when a charge is applied to a graphite electrode submerged in sulfuric acid. However, the specifics of how these oscillations affect the structural transformation of graphite remained an enigma until now.
The pivotal moment in unraveling this chemical conundrum came through groundbreaking synchrotron techniques, which enabled researchers to perform X-ray diffraction scans at incredible speeds. This technological advancement allowed them to capture the dynamic changes in material structure during the oxidation process. Much to their surprise, the experiments showcased an intermediate phase that would emerge, vanish, and then reappear at various points throughout the oscillation cycle, creating a repeating pattern of structural transition. This unexpected observation led the research team to consider the existence of a novel type of oscillating reaction, one that had never before been documented in the realms of chemistry.
The implications of this discovery extend beyond the immediate findings of oscillation. The researchers’ insights contribute to a broader understanding of chemical kinetics and provide a framework for examining reaction mechanisms in both organic and inorganic chemistry. This is particularly significant as the nature of oscillating reactions, previously confined mainly to biological systems, is now recognized within the inorganic chemical landscape. As noted by Professor Alexandr Talyzin, the depth of the research blossomed from what was initially a focused study into a rich field of investigation, promising ongoing exploration into the principles governing oscillating reactions.
The significance of this new oscillating reaction cannot be overstated within the context of chemical history. Oscillating reactions have long fascinated scientists, culminating in Ilya Prigogine receiving the Nobel Prize in 1977 for pioneering work that illuminated the transition from disorder to order within chemical processes—one of the landmarks of non-equilibrium thermodynamics. By building upon the foundations laid by previous scholars, the contemporary analysis of oscillation within the diamond of graphite oxide formation reinforces the idea that there is still much to learn about chemical behavior.
As the scientific community integrates these findings, there is a hopeful anticipation that new theories could be developed to further elucidate the mechanisms behind this oscillating reaction. The hope is not only that this unique behavior will prompt the exploration of analogous reactions but also that it will redefine the approach to understanding complex chemical interactions. As Talyzin emphasizes, this discovery may usher in an era of exciting revelations in chemical reactions, challenging existing paradigms and fostering a new understanding of oscillation dynamics.
The unveiling of this puzzling chemical reaction serves as a reminder of the ever-evolving nature of science and the importance of inquiry. The work completed at Umeå University has not only provided answers but has ignited a conversation surrounding the fundamental principles of chemistry. This discovery highlights that each breakthrough in scientific research may lead to questions yet unanswered, perpetuating a never-ending quest for knowledge in the intricate world of chemistry.

Leave a Reply