Helices are fundamental motifs found in various crucial biological macromolecules, particularly proteins. Their distinctive winding shape is not merely an aesthetic feature; it plays a pivotal role in determining the functionality and behavior of these proteins within living organisms. This characteristic twist is influenced by the specific arrangement of their constituent building blocks, setting the groundwork for the intricate dances of molecular interactions that sustain life. A deeper comprehension of helix formation can elucidate the structural biology of proteins and their operational principles, paving the way for advancements in biochemistry.
At the heart of helix formation lies the concept of chirality, which refers to the geometric property where a molecule cannot be superimposed on its mirror image. In the realm of peptides—short chains of amino acids—the helical conformation emerges through the precise repetition of these amino acids. This repetition is not trivial; it creates a chiral layer that profoundly influences the properties and biological activities of the peptide. Understanding this relationship allows scientists to explore the nuances of peptide structures and their resultant functions in cellular processes.
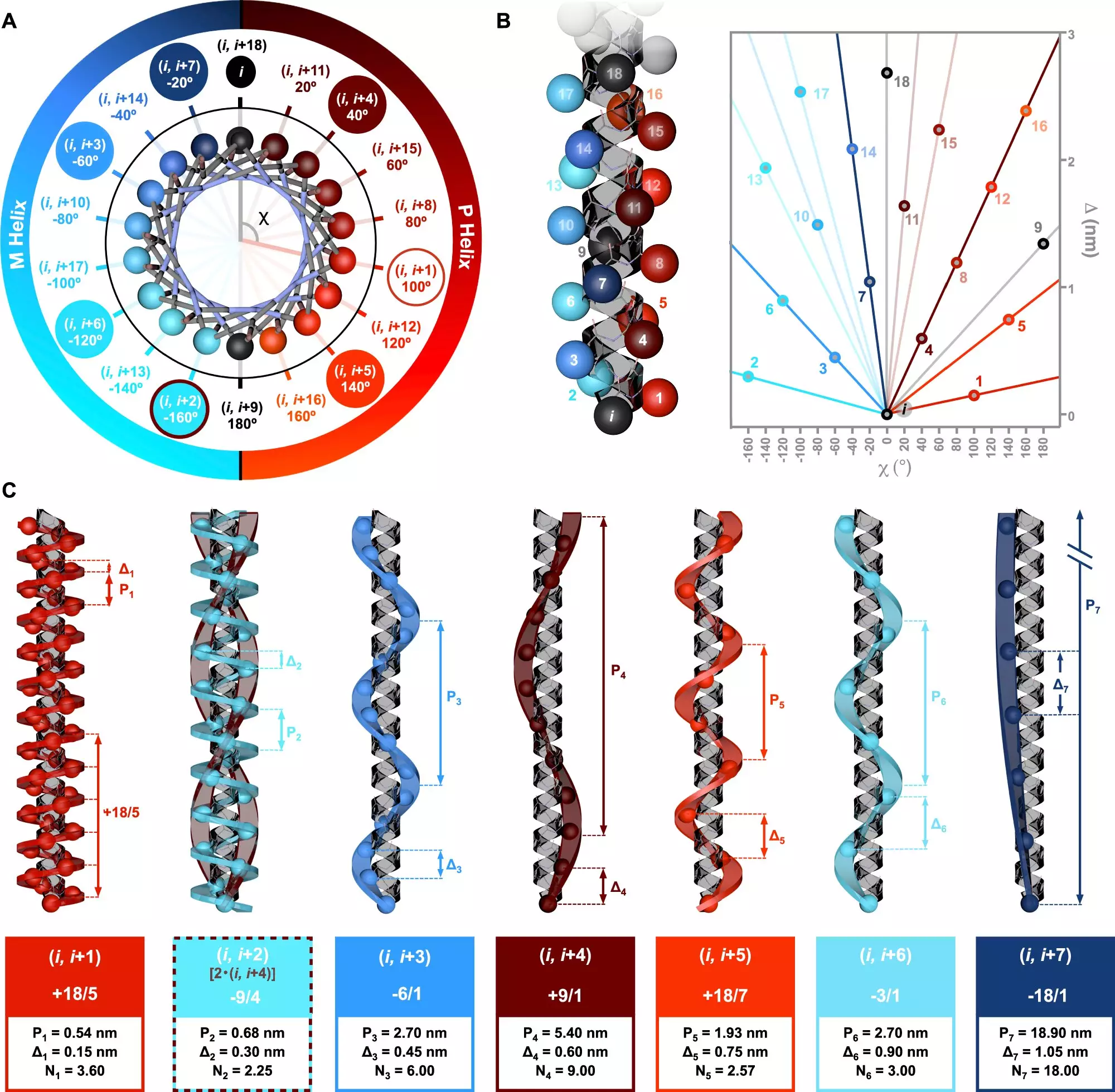
A recent paper published in Nature Communications delves into the intricate chiral nuances of alpha-helical peptides, revealing groundbreaking insights into their symmetry and structural properties. Spearheaded by Dr. Julián Bergueiro from the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), the study presents a novel symmetry model that articulates the relationship between different types of helices and their corresponding amino acid sequences. Through sophisticated computational methods and circularly polarized light spectroscopy, the research team was able to assess how specific arrangements of amino acids give rise to diverse helical topologies.
The importance of these findings extends well beyond theoretical curiosity. Dr. Bergueiro notes that the different patterns in amino acid arrangements lead to distinct helical structures that are integral to the functions of numerous proteins. This understanding opens doors to innovative applications in medicine and biotechnology. Imagine being able to design new molecular architectures tailored for specific therapeutic roles or biocompatible materials with enhanced efficacy. By strategically controlling the sequences of amino acids—termed monomers—scientists could engineer custom polymer topologies, representing a transformative shift in macromolecular engineering.
This study not only enhances our understanding of peptide chemistry but also contributes to a broader perspective on how helical structures can be systematically exploited for future scientific advancements. As researchers unlock the complexities of helical formations, they build a foundation for novel compounds and technologies that could revolutionize various fields, from pharmaceuticals to materials science. The potential applications are immense, and as the study demonstrates, controlling helical structure through amino acid sequence offers exciting prospects that promise to change the landscape of molecular engineering in the years to come.

Leave a Reply