The synthesis of chemical compounds often presents challenges that can limit the efficiency and effectiveness of producing essential molecules. Recently, researchers at the University of Illinois Urbana-Champaign have made strides toward overcoming these challenges by developing a novel catalyst inspired by natural enzymes, significantly enhancing the synthesis of ethers. This groundbreaking research, led by Professor M. Christina White, presents a transformation in the field of organic chemistry with implications for drug development, food production, and personal care products.
Ethers are foundational elements found in a broad range of chemical products. Their unique structure plays critical roles in various applications, from pharmaceuticals to everyday consumables, making them indispensable in both industrial and consumer sectors. Traditional methods of synthesizing ethers often involve complicated procedures that require substantial quantities of raw materials and can yield a diverse array of byproducts, complicating the purification process. The urgency to develop more effective and efficient synthesis methods grows as the demand for complex organic molecules increases in modern chemistry.
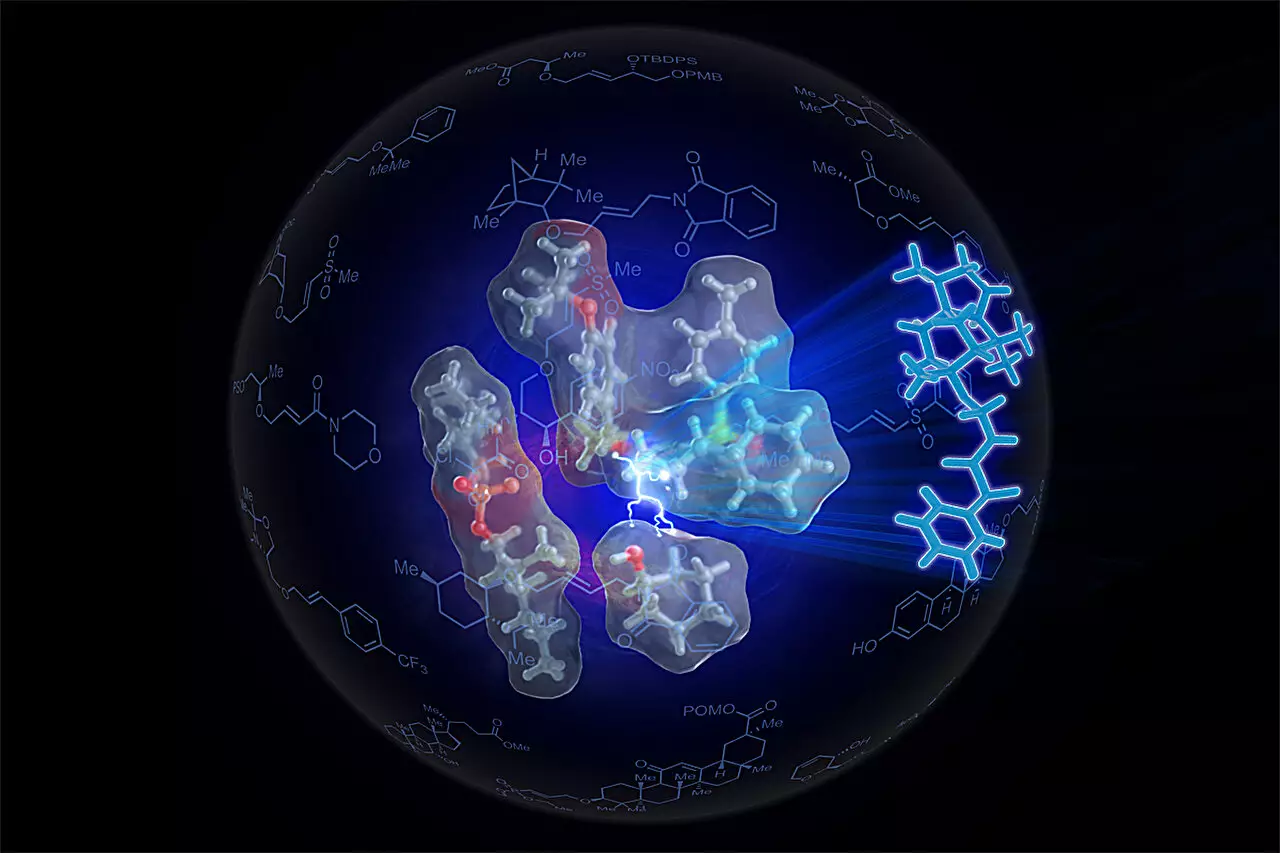
The innovative catalyst developed by the University of Illinois team, termed the SOX catalyst, leverages inspiration from nature’s finest chemists: enzymes. Enzymes are biological catalysts that facilitate various chemical reactions in living organisms, demonstrating remarkable efficiency and specificity. Recognizing the organization and positioning strategies that enzymes utilize to expedite reactions, the researchers crafted a self-assembling small-molecule catalyst designed to optimize the synthesis of ethers by tightly positioning reactants.
Graduate student Sven Kaster, the primary author of the research, articulated the challenge: conventional ether synthesis requires a significant activation step, leading to unwanted byproducts. By pivoting from traditional methods and instead focusing on enhancing the natural reactivity of alkenes and alcohols through superior alignment, the team effectively reduced the necessary reactant amounts while improving yields.
The Mechanism Behind the SOX Catalyst
At the heart of this novel approach is the use of palladium, a transition metal notorious for its unique catalytic properties. The SOX catalyst facilitates the cleavage of the carbon-hydrogen bond in alkenes, allowing them to interact with alcohols more readily, all while minimizing undesirable byproducts. The significant innovation lies in the catalyst’s specific geometry and electronic properties, realized in its advanced form, Sven-SOX. This catalyst excels in ensuring the precise proximity and orientation of the reactants, much like a well-coordinated dance, leading to successful ether creation.
The process not only streamlines the synthesis of ethers but also enables the formation of complex ethers that were challenging to create using conventional methodologies. This ability to generate more than 130 different ethers, including those containing bulky components, demonstrates the expansive potential for future applications in synthetic organic chemistry.
The implications of the research extend beyond merely increasing the efficiency of ether synthesis. The mild conditions required by the SOX catalyst stand out as an advantage, enabling the inclusion of sensitive functional groups that would ordinarily be vulnerable to degradation in harsher environments. The researchers assert that the procedure could potentially be simplified to a level accessible to novice chemists, illustrating both the practicality and educational potential of their work.
The environmental benefits associated with reduced waste generation and the need for fewer reaction steps further highlight the significance of this research in meeting contemporary scientific and ecological demands. This innovation not only reflects cutting-edge advancements in chemistry but is also a step towards sustainable and responsible chemical production.
Looking forward, Professor White and her team aspire to explore additional small-molecule catalysts designed to mimic the activity of enzymes, potentially opening avenues for synthesizing a variety of other chemical classes. Their work exemplifies the integration of biological principles into chemical synthesis, setting a precedent for future research endeavors.
This leap in ether synthesis methods exemplifies a broader narrative in science regarding the power of collaboration between disciplines—where nature’s complexities inspire humanity’s quests for innovation. The study, recently published in the journal Science, underscores the critical role of foundational research and collaboration in driving advancements in chemistry that can reshape industries and promote a more sustainable future.
The efforts by the University of Illinois team signify not only a remarkable scientific achievement but also a changing paradigm in how chemists can approach synthesis challenges. By forging a path based on natural inspiration and innovative thinking, they are paving the way for future advancements that will resonate throughout the scientific community.

Leave a Reply