Proteins are essential molecular components of life, carrying out a multitude of functions within cells. In order to fulfill their roles effectively, proteins must adopt specific three-dimensional structures. This structural integrity is crucial for their functionality, much like the components of a well-designed machine.
Chaperonin complexes play a vital role in assisting newly synthesized proteins in achieving their correct three-dimensional shape. These complexes act as folding helpers, ensuring that proteins fold into their functional form. Misfolded proteins can lead to severe diseases such as Alzheimer’s and Parkinson’s, highlighting the significance of understanding the mechanisms of chaperonin complexes.
Researchers at the MPI of Biochemistry in Martinsried and the University Medical Center Göttingen utilized cryo-electron tomography (cryo-ET) to examine chaperonin complexes in the bacterium E. coli. This cutting-edge technology allowed them to observe protein folding processes with unparalleled detail, capturing conformational changes within the chaperonin complexes as they interacted with client proteins.
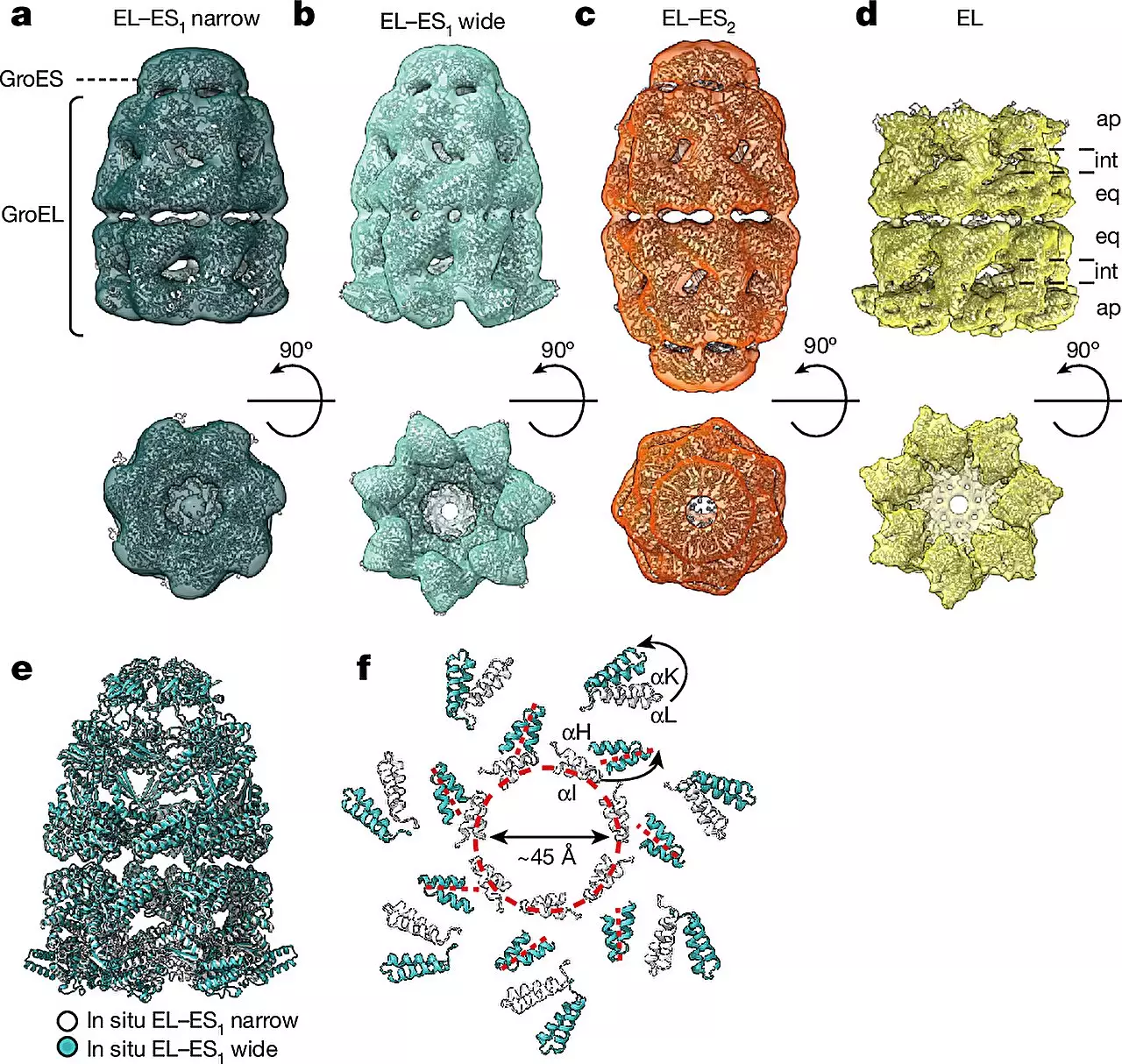
The study revealed the presence of two main forms of the GroEL-GroES chaperonin complex within bacterial cells – the “bullet” and “football” shapes. The bullet form featured a GroES cap attached to only one side of the GroEL barrel, while the football form exhibited symmetrical structural characteristics. These findings shed light on the dynamic nature of chaperonin complexes and their role in protein folding.
Advancements in Cryo-Electron Microscopy
The integration of cryo-ET with single-particle cryo-electron microscopy (cryo-EM) and quantitative mass spectrometry allowed researchers to visualize chaperonin complexes in different cellular states. This approach provided insights into the diverse conformations of chaperonin complexes and their abundance within living cells. By studying chaperonin complexes directly within the cellular environment, researchers overcame limitations faced by in vitro experiments, offering a more accurate representation of protein folding processes.
Understanding the structure and function of chaperonin complexes can pave the way for the development of novel therapeutic strategies for diseases associated with protein misfolding. By elucidating the intricate mechanisms of chaperonin-assisted protein folding, researchers aim to identify potential targets for drug intervention, particularly in conditions like Alzheimer’s and Parkinson’s disease.
The study’s findings underscore the versatility and adaptability of chaperonin complexes during the protein folding process. By unraveling the dynamic interplay between chaperonins and client proteins, researchers have gained valuable insights into the molecular mechanisms that underpin cellular function. This research not only contributes to our fundamental understanding of protein folding but also holds promise for the development of innovative therapeutic approaches in the realm of molecular biology.

Leave a Reply