The development of lithium-metal batteries holds great promise for the future of battery technology, as they have the potential to significantly surpass the energy densities of lithium-ion batteries currently dominating the market. However, one of the main challenges facing lithium-metal batteries is their limited lifespan. In a recent study conducted by researchers at the University of Science and Technology of China and other institutes, a new electrolyte design was introduced that aims to address this issue and pave the way for highly performing lithium-metal pouch cells with longer lifespans.
One of the major obstacles in the widespread adoption of lithium-metal batteries is their short cycle life, which is currently limited to approximately 50 cycles. This is in stark contrast to commercial lithium-ion batteries, which can typically retain their performance for around 1,000 cycles. The primary reasons behind this limitation are the growth of lithium dendrites, the high reactivity of lithium-metal, and the degradation of the electrolyte, all of which contribute to the constant degradation of the battery during operation.
To combat these challenges, Prof. Shuhong Jiao and her colleagues designed an electrolyte that can stabilize both the anode-electrolyte and cathode-electrolyte interfaces in lithium-metal battery cells, thus suppressing the degradation of the electrolyte and extending the lifespan of the battery. This innovative electrolyte design builds upon previous research into the microscopic processes within lithium-metal batteries and aims to provide a cost-effective solution using readily available components.
A New Class of Electrolytes
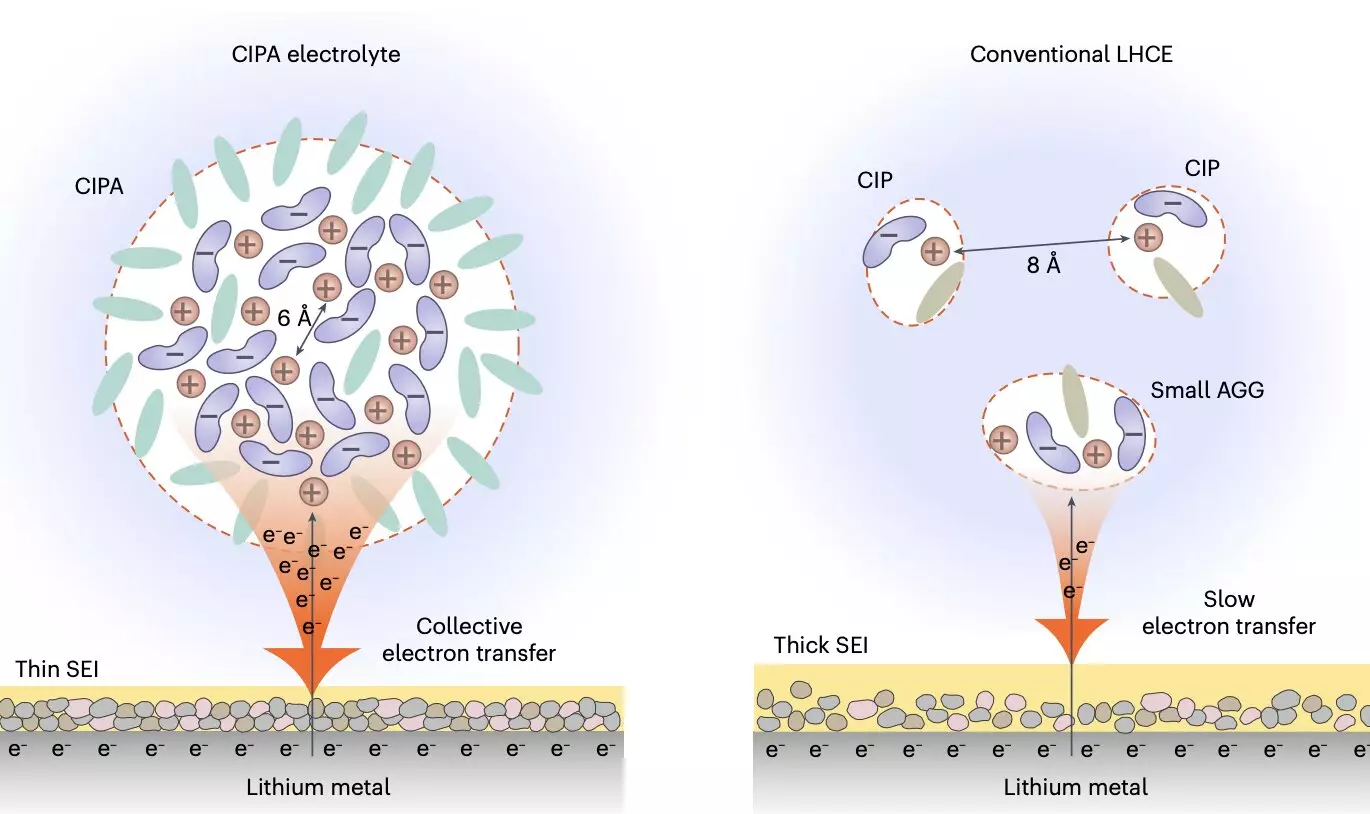
Through collaborative efforts with other research teams, Prof. Jiao’s group developed a new class of electrolytes characterized by a unique solvation structure. This mesoscopic level tuning of the electrolyte’s structure focuses on the interaction between ion pairs that form the electrolyte’s aggregate structure, resulting in the creation of compact ion-pair aggregates (CIPA). This innovative design has the potential to revolutionize electrolyte development for lithium-metal batteries and improve overall battery performance.
Enhanced Stability and Performance
The newly designed electrolyte exhibits collective reduction on the lithium-metal anode, which leads to the rapid reduction of anions on the surface of the lithium and the formation of a stable solid-electrolyte interface (SEI). This process suppresses the decomposition of the electrolyte and promotes homogeneous lithium deposition, ultimately improving battery performance and stability. Additionally, the electrolyte demonstrates good oxidative stability and suppresses the dissolution of transition metal elements from the cathode, further enhancing the stability of the cathode interface.
A Promising Future
In initial tests, the researchers used the newly designed electrolyte to create a 500 Wh/kg lithium-metal pouch cell that retained 91% of its energy after 130 cycles. This promising result underscores the potential of the new electrolyte design to significantly extend the life of lithium-metal batteries and unlock new possibilities for higher energy densities and longer lifespans. Moving forward, further research is needed to assess the full potential of this innovative electrolyte and explore its application in other battery systems to achieve even higher energy densities and longer cycle lives. By continuing to push the boundaries of electrolyte design, researchers can pave the way for the widespread adoption of lithium-metal batteries and the next generation of battery technology.

Leave a Reply